75
Glorious Years of Spectroscopy at Banaras Hindu University
S. N. Thakur, Ex-Professor of Experimental
Spectroscopy, BHU
1.
Introduction
The foundation stone of
Banaras Hindu University was laid down on the auspicious day of Vasant Panchami, 1916 and the Department
of Physics was established on the same historic occasion. The tradition of
research in the field of Spectroscopy was initiateded by Professor R K Asundi
in 1939. This
achievement was a crucial milestone in the evolution of BHU as Sarva Vidya
Ki Rajdhani.
It is a very pleasant coincidence that the diamond jubilee of reaching
this milestone falls in 2014, two years before the centenary year of the
university itself which is to be celebrated in 2016. It is also noteworthy that
in October 2012, the UNESCO Executive Board at the
organization’s Headquarters in Paris adopted a resolution proclaiming 2015 as
the International Year of Light and Light-based Technologies. Life without
light is simply beyond imagination. The role of light in human history, education,
science & technology, sustainability and development of culture has been
profound. Even in ancient Indian scriptures dating back to BCE, the importance of light in human development is highlighted. The Brihadaranyak Upanishad metaphorise knowledge as
light in the shloka - ... Tamaso Ma Jyotirgamaya .... -
[Oh Almighty,] ... lead us from darkness to light ... .While persuading the
Kashi Naresh to donate some land on the bank of river Ganga for the University, the Banaras Hindu University founder Mahamana Pt Madan Mohan Malviya is said to have convinced the reluctant Maharaja
saying: “Your Excellency! Imagine
thousands of students of the University worshipping the rising Sun on the western bank of the holy river
which you would be pleased to watch from the Ramnagar Fort.”
Pt.
Madan Mohan Malaviyaji (1861- 1946)


Professor N. L. Singh (1909- 1996)
More and more hard working and bright students joined spectroscopy because of the very special mode of student-teacher interaction in this group and by mid 1940s their number became so large that two-room space was not enough to accommodate all the research scholars. Prof. Asundi wrote to the university authorities for more space for his group in the physics department but there was no immediate solution to this important requirement. By the late 1940s, however, the Intermediate Science classes were shifted to Central Hindu College at Kamachha and the then Vice-chancellor Pt. Govind Malviya, impressed by the research atmosphere of spectroscopy laboratory, permitted the vacant space to be occupied by Prof. Asundi and his group and to make use of the furniture that were left behind. The students of spectroscopy laboratory by this time included some of the very bright ones like Jagdeo Singh, D D Pant, D P Misra, Lalji Lal, P Venkateswarlu, R M Singh, M R Padhye, K S Adiga and N A Narasimham, who brought much glory in the later years as mentioned in the introductory section of this article. These young and bright research scholars gladly offered their services for the hard manual work that was required to create suitable spaces, by rearranging the big wooden almirahs and long heavy tables in the large halls, to form laboratory, lecture room and sitting places for them as well as for the teachers. As a result of unique cooperation between bright research students and teachers, researches carried out in spectroscopy were of such high quality that research grants were generously sanctioned by the university as well as by the government agencies. The head of the department, however, did not miss any occasion to hinder the rapid pace at which research activities were progressing in spectroscopy and this fact became a common knowledge at the highest level of university administration. Acharya Narendra Deo, as Vice-Chancellor, decided to put an end to this man made hindrance in the progress of research work and in 1953 the spectroscopy section was given the status of a full fledged Department separate from the Department of Physics and Prof. Asundi was made the Head of the Department. Laboratories of the Department of Spectroscopy were kept open practically 24 hours and research scholars worked very hard under the inspiring guidance of Prof. Asundi and Dr Singh.

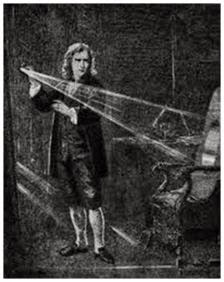
The science
of spectroscopy was conceptualised by Sir Isaac
Newton in 1666 with the formation of spectrum by passing the sunlight through a
glass prism as illustrated in Fig.1. Very significant contributions in this
important field have been made by Indian scientists and some of them - e.g.
Raman Effect, Saha’s thermal- ionization and Joshi effect- have played crucial
role in the development of science and technology of light.

Fig.1.
In a darkened room, Newton made a slit in his window shutter to admit a ray of
light. Seeing the array of colors cast by a prism, he carefully analyzed the
distinct reflections and refractions of colors that led to the science of
Spectroscopy in 1666.
Spectroscopy
laboratory reflects how the university has
contributed in the making of a modern, erudite India as well as in its
own growth
into one of the nation's best educational institutions, just as Pt
Madan Mohan Malaviya had envisaged. According to one of the popular anecdotes related to
Malaviyaji’s dream of establishing a university in Varanasi, Sir Sunderlal, a
prominent lawyer in Allahabad in late 1880s, advised him to first start up a
school and then endeavour to upgrade it into an
university. Malaviyaji is reported to have told the learned jurist
with utmost respect that he would build an university only and be back to him
offering the post of its first Vice-Chancellor. This relationship has been
monumentalised in the form of Sir Sunderlal Hospital in the
university which was built in the honour of its first Vice-chancellor. The story was repeated in a different manner
in the establishment of spectroscopy research in the Physics department in 1939
when Professor S S Joshi, the then principal of Science College and one of its
brightest alumni, tried to create an atmosphere of research that the college lacked for almost 25 years
of its existence. The university appointed Dr Asundi in the vacancy created by
the retirement of Professor P K Dutta in 1938. This was against the will of the
then Head of Physics and he continued to put hurdles like providing inadequate infrastructure for the spectroscopy lab - only two spare rooms with one table and a rotating wooden chair
and a discarded physical balance [1]. The laboratory started with an improvised
Constant Deviation Glass spectrometer and a commercial Hilger Medium Quartz
Spectrograph with the Bunsen burner flames as the sources of light to
investigate the spectra of atoms and molecules. This tradition of improvising
instruments and acquiring the state-of –art commercial equipments through
research grants continued till late 1980s when importing costly Lasers and
Spectrometers was extremely difficult. Brilliant students from spectroscopy joined several major universities and laboratories across the country and
added further excellence in the teaching and research.
Professor Jagdeo Singh and Professor Devi Dutt Pant were from the
first batch of spectroscopy at BHU. Professor Singh went to Imperial College, London
for his doctorate degree in Geophysics and played a major role in introducing Geophysics as a separate discipline in BHU. He
later joined the Indian School of Mines, Dhanbad and went on to establish the Geophysics in the Department of Physics in 1950s. Professor Pant
was the first to initiate investigations in
Time-resolved spectroscopy at Nainital in 1950s and created a famous center of
Ultra-fast spectroscopy at the Kumaun University. Later Professor P
Venkateswarlu carried the tradition of spectroscopy to IIT Kanpur and
established one of the first major centers of Laser-spectroscopy while
Professor M R Padhye carried out excellent work in Molecular spectroscopy at
Chemical Technology in Bombay University. Professor Trilochan Pradhan developed
research groups in Orissa and retired as the Director of Institute of Physics,
Bhubaneshwar. Dr S N Garg was involved
in the application of spectroscopy in Forensic science and went on to become
the Director of Central Forensic Laboratory at Hyderabad, while Dr N A Narasimham
went on to become Head of the Spectroscopy Division at the Bhabha Atomic
Research Center. While Professor B. Bhattacharya created excellent teaching and
research facilities in the Physics Department of Jadavpur University, his
younger brother Professor B N Bhattacharya started Microwave Spectroscopy at IIT
Bombay and was one of its most creative Head of the Physics Department. Thus an
excellent network of spectroscopy research was created by the first generation
of students in this field from BHU who knew the lack of equipment in their alma
mater and extended all possible help with great affection to the later
generations of research students, from 1960 till late 1970s, to work in their
well furnished laboratories. This institutional networking led to rapid
developments in spectroscopy at BHU in a manner which can be compared in recent
times by spread of knowledge across the world through the Internet. Some of the
man made hurdles led to the creation of a Spectroscopy Department by its separation
from the Physics Department in 1953 and the recognition of holistic development
of physics led to its merger again with the latter by early 1970s. It is
relevant to emphasize that the flow of research was never hampered during its
brief separate identity; in fact the tempo of growth was more vigorous. Dr
Lalji Lal, a first generation spectroscopist from BHU joined the Udai Pratap
College in Varanasi in early 1940s and established an excellent laboratory for
the undergraduate students some of whom come to BHU for higher studies and a
cordial environment for the exchange of knowledge has continued till date. Even after the upgradation as an autonomous college and creation of facilities for
research at this famous institution the person to person cooperation has been
very helpful to a wider population of students specializing in spectroscopy.
The spirit of improvisation and cooperation, to acquire knowledge through
research, that was started more than seven decades ago in the spectroscopy
laboratory of BHU, still continues. This is a very pleasant and satisfying
experience for me to realize that the traditions decay very slowly. In the
following sections I would try to present a historical perspective of
Spectroscopy and Lasers, as I have come to know from my long association with
this center of knowledge, with mentions of its contributions in the field of human resource development as well as in
science and technology.
2.
Low Resolution Emission Spectroscopy of Flames and Electric Discharges
(1939-60)
The first phase of spectroscopic
investigations were directed by Professor R K Asundi from 1939 till his
retirement in 1956. Professor Asundi was actively supported by
Dr N L Singh who had spent more than 6 years, before arrival of the former in
BHU, in acquiring skills of experimental and theoretical research under the
guidance of Professor C M Sogani and Professor V V Narlikar at BHU and under
Professor K S Krishnan at Calcutta. Emission Spectra of diatomic molecules BO,
CuCl, CuBr, and CuI were recorded on the medium quartz spectrograph using
excitation in flames from Bunsen burner suitably enhancing their intensity by
oxy and oxy-acetylene flames whenever so required. Facilities at a local
malleable casting workshop at Assi in Varanasi were used under the direction of Dr N L
Singh to cast stands and components of electric arc sources as well as
components for assembling prism and grating spectrographs. A number of
discharge tubes and high frequency oscillators were designed and fabricated
with the meager glass blowing and electronic workshop facilities of the physics
department to investigate the spectra of gases and vapours. Gradually
comparators were designed and fabricated using a travelling microscope for the
measurements of spectral lines and projectors as well as viewers were assembled
with appropriate lamps for visual inspection of the spectra recorded on
photographic plates and films. Dr N L Singh guided Shri Rajnath to become an
extremely competent glass blower who could make discharge tubes fitted with
metallic electrodes that were leak proof under high degree of evacuation needed
for excitation of molecules in the gas phase. Looking back from the existing
facilities in the Laser and Spectroscopy Laboratory it is beyond our wildest
imagination to comprehend the amount of patience and toil by the teachers, the
students and the supporting staff that went into the making of this world
renowned laboratory. This was in addition to the class room teaching of the
intricacies of atomic and molecular structure. In the words of Professor N L
Singh, “While learning more and more of the subject, I would direct the
students in performing experiments and would teach them theory as well with
Prof. Asundi sitting by their side. It used to be an unique class. Thus I came
to be addressed as ‘Master Saheb’ and Prof. Asundi as ‘Doctor Saheb’. This
sweet epithet has clung to me lifelong” [1].

Professor R. K. Asundi (1895- 1982)
Professor N. L. Singh (1909- 1996)
More and more hard working and bright students joined spectroscopy because of the very special mode of student-teacher interaction in this group and by mid 1940s their number became so large that two-room space was not enough to accommodate all the research scholars. Prof. Asundi wrote to the university authorities for more space for his group in the physics department but there was no immediate solution to this important requirement. By the late 1940s, however, the Intermediate Science classes were shifted to Central Hindu College at Kamachha and the then Vice-chancellor Pt. Govind Malviya, impressed by the research atmosphere of spectroscopy laboratory, permitted the vacant space to be occupied by Prof. Asundi and his group and to make use of the furniture that were left behind. The students of spectroscopy laboratory by this time included some of the very bright ones like Jagdeo Singh, D D Pant, D P Misra, Lalji Lal, P Venkateswarlu, R M Singh, M R Padhye, K S Adiga and N A Narasimham, who brought much glory in the later years as mentioned in the introductory section of this article. These young and bright research scholars gladly offered their services for the hard manual work that was required to create suitable spaces, by rearranging the big wooden almirahs and long heavy tables in the large halls, to form laboratory, lecture room and sitting places for them as well as for the teachers. As a result of unique cooperation between bright research students and teachers, researches carried out in spectroscopy were of such high quality that research grants were generously sanctioned by the university as well as by the government agencies. The head of the department, however, did not miss any occasion to hinder the rapid pace at which research activities were progressing in spectroscopy and this fact became a common knowledge at the highest level of university administration. Acharya Narendra Deo, as Vice-Chancellor, decided to put an end to this man made hindrance in the progress of research work and in 1953 the spectroscopy section was given the status of a full fledged Department separate from the Department of Physics and Prof. Asundi was made the Head of the Department. Laboratories of the Department of Spectroscopy were kept open practically 24 hours and research scholars worked very hard under the inspiring guidance of Prof. Asundi and Dr Singh.
2.1
Spectroscopy of Diatomic and Polyatomic Molecules
Prof. Asundi, prior to
his joining the Banaras Hindu University, had made significant contributions to
the study of diatomic molecular spectroscopy and it was, therefore, natural to
record emission spectra of diatomic species and elucidate their structure and
dynamics. The molecules investigated during the early days included Hg2,
HgI, CuI and I2. Emission spectra of iodine vapor recorded by
Venkateswarlu [2] revealed a new band system in the region 2785 to 2750A in
addition to a large number of new bands in the 3455 - 3015A system, 2730 -
2520A system and in the 4420-4000A system reported by earlier workers. The
absorption bands data in the region 2000 -3500A were discussed in the light of
the information from the emission spectra. Prof Venkateswarlu retained a
lifelong interest in the electronic spectra of halogen molecules and the study
of the resonance series in the halogen dimer with successively higher
resolutions in later years has enabled very precise values for their
dissociation energies.
The electronic spectroscopy of polyatomic molecules gets
more complicated due to the presence of many normal modes of vibrations (3N-6
and 3N-5 for nonlinear and linear polyatomic molecule respectively made of N
atoms). Delicate experimental techniques were developed to record emission
spectra of highly fragile aromatic hydrocarbons using Tesla coil discharge,
transformer discharge with internal electrodes in the discharge tube and with
high frequency (1.15 MHz) discharge with external electrodes. Padhye [3] made a
systematic study of the 260 nm emission spectrum of benzene excited by a
transformer discharge through flowing benzene vapour. The intensity
distribution amongst different progressions was found to be substantially
different from that in the fluorescence spectrum and it was accounted in terms
of the excited state Boltzmann factor and the Franck-Condon principle. Fermi
resonance between two vibrational levels 1596 cm-1 and (606+992) cm-1
was observed up to four members of the progression due to the ring breathing
vibration (992 cm-1). These results provided valuable data on the
vibration-vibration interactions for a polyatomic molecule.
The 260 nm electronic transition in benzene is symmetry
forbidden but vibronic bands are observed due to Herzberg-Teller intensity
stealing process although the origin band (000) is not
observed. A systematic study of the electronic transitions in mono- and
di-substituted benzenes was carried out during 1950s [4-7] and the experimental
information obtained from these investigations can be summarized as follows.
· The relative intensities of the
electronically allowed bands and the vibronically induced ones were obtained by changing the chemical nature of the substituent and their substitution sites
on the carbon ring.
· The frequency shifts of the 000
bands in substituted benzenes from that of the estimated position of 000
band of benzene were obtained.
· The changes in vibrational frequencies of
several normal modes, in going from the ground state to the excited electronic
states, of substituted benzenes were obtained.
· The relative intensities of the
background continuum in the emission spectra of methyl substituted benzenes
were noted to be the most prominent
These studies provided
data on electronic interaction between the substituent replacing a hydrogen
atom and electrons of the carbon ring of benzene and observed energies of
excited electronic states were compared with those calculated from
semi-empirical theories. Thus a valuable insight into the electronic structure
of substituted benzene molecules could be obtained.
During 1954-56 Prof. N L Singh visited North America and
worked in the Spectroscopy Laboratories of Duke University, University of North
Carolina, Massachusetts Institute of Technology in USA and the National
Research Council of Canada. This visit not only brought him abreast of the
latest trends in spectroscopic research, but he also brought back a large number
of photographic plates, recorded by him under high resolution, to be analyzed
and interpreted at BHU. Prof. Asundi retired from university service in 1956 and
went to Bhabha Atomic Research Center to establish experimental facilities in
the Spectroscopy Division for analysis of nuclear fuel and related materials.
Prof. Singh was appointed as Head of Department of Spectroscopy in BHU and had
the excellent company of Dr S N Garg, Dr I S Singh, Dr K N Upadhya, Mr C M
Pathak as teaching staff and D K Ghosh, R N Singh, S P Singh, R J Singh, S K
Tiwari, D K Rai, M G Jaiswal, R B Singh, S N Thakur, and many others, as
research scholars, who worked enthusiastically with unique cooperation towards
latest developments in the fields of Atomic and Molecular structure. A new one
year course of Post Graduate Diploma in Spectroscopy was started to train
manpower for the benefit of spectro-chemical analysis of samples in various
laboratories of the university and also for the technical staff at the Diesel
Locomotive Workshop in Varanasi and a similar unit in HINDALCO at Mirzapur. The 1
hour lecture and 2 hour laboratory classes were run in the evening for the
convenience of the technical staff who worked in their respective labs during
the daytime. This course not only became very popular among research students
in the area of chemical, metallurgical, medical and biological sciences but also a few scholars from the
Department of Ancient History and Culture joined with the intention of knowing
the chemical constitution of ancient objects. One can only appreciate the
vision of Professor Singh who used his experience of visiting foreign
laboratories to further the cause of, not only science education in the
impoverished regions of our country but also, the technical and industrial
development.
On the occasion of the 50th anniversary of
Spectroscopy at BHU, we received a large number of messages from leading
international scientists who had visited the spectroscopy laboratory and some
pertinent comments are reproduced below [1]:
Nobel
Laureate Dr Gerhard Herzberg, from Canada wrote, “I am glad to send my best wishes
to the Spectroscopy Laboratory of the Banaras Hindu University on its fiftieth
anniversary. Professor R K Asundi was a close friend who by his own original
research and by that of his students contributed greatly to the development of
science in India. I remember very well my visit to the Spectroscopy Laboratory
in 1958 and came to admire the spectroscopic work that was done there under
difficult circumstances.”
Dr
Manfred Stockburger of Max-Planck Institute at Gottingen wrote, “First of all I
am sending my best wishes for celebrating the 50th anniversary of
foundation of the Spectroscopy Laboratory at Banaras Hindu University.
Personally I became familiar with the work from this laboratory when in the
period 1954 and 1962 I was a student and research fellow in Professor Schuler’s
institute in Germany. I remember the beautiful emission spectra of organic
molecules, in those days fixed on photographic plates, which were published by
Professor Asundi’s and Professor Singh’s groups. Indeed we had common interest
in studying by spectroscopic methods the photophysical behavior of molecules
like benzaldehyde using a so-called Schuler discharge tube.”
3.
High Resolution & IR Spectroscopy, Potential Functions, Quantum Chemistry
(1960-75)
Professor N L Singh had
carried out studies on two diatomic molecules OD and PO during his brief stay
in the spectroscopy laboratories of USA and Canada. The spectrum of OD in the
300 nm region was recorded in the presence of a constant magnetic field so the
rotational lines of the various branches showed a Zeeman splitting. The
analysis of this spectrum was published much later [8]. The PO spectrum was
recorded on the 10.6 m grating at NRC, Canada and a very large number of bands
of the B-X system (the so called β system) were rotationally analyzed. While
Professor Singh had published an analysis of a few bands involving small values
of vibrational quantum numbers v’ and v” [9], his photographic plates,
containing a very large number of bands, served as a valuable treasure house
for the extensive studies on this system [10, 11].
Prior to 1962 the laboratory had
only a 6.6 m grating spectrograph in the Eagle mount with a 15 cm long grating
of 600 grooves per millimeter having a dispersion of 1.2A/mm in the first
order. This instrument facilitated the search for novel molecular species and
newer electronic transitions. The studies involved the use of different types
of excitation sources e.g. uncondensed and condensed transformer discharge,
high current D.C. discharge and microwave discharge. The availability of a 10.6
m grating with 1200 grooves/mm required a herculean effort on the part of R B
Singh and S K Tiwari to set up (see Fig. 2) the most sophisticated spectrograph
in the laboratory in the Eagle mount [12].
Fig.2 The light-proof darkened wooden housing for 10.6 m grating spectrograph with the slit and photo-plate assembly at the far end of 15 meter long room (left) and the concave grating on rails for its linear as well as angular adjustments (right)
Fig.2 The light-proof darkened wooden housing for 10.6 m grating spectrograph with the slit and photo-plate assembly at the far end of 15 meter long room (left) and the concave grating on rails for its linear as well as angular adjustments (right)
Fig.3 Rotational structure in the (0,0)
band of B2Σ→X2Π3/2 transition in SbO molecule
Around 1960 a series of
research papers, on the spectrum of SbO molecule, were published by several
workers from Andhra University, Waltair (India) and from Japan. This molecule
is isoelectronic with PO and its B-X system was expected to be analogous to the
β system of PO. The analysis published by the Waltair group confirmed this but
did not reveal the isotopic doubling arising from the two almost equally
abundant Sb isotopes. This lacuna was removed in one of the first
investigations of high resolution spectroscopy of SbO using the 10.6 m grating
spectrograph [13]. It was later shown from the analysis of B2Σ→X2Π3/2 subsystem
(Fig. 3), that the B2Σ state
has an appreciable spin splitting[14].The AsO and AsO+
molecules being intermediate between PO and SbO were also explored but their
toxic nature prevented extensive investigations on them [15-16].
Copper halides molecules are fairly simple, analogous to
alkali halides, but the presence of a closed 3d shell in copper atom with one
electron energies comparable to that of the 4s orbital gives rise to a much
richer energy level scheme in Cu-halides. It was found that an earlier
rotational analysis of CuI published from BARC group in India was incorrect
since the ground state rotational constants obtained from it failed to satisfy
several empirical rules [17]. A detailed investigation of CuI spectra was
carried out to analyze rotational structures in several electronic transitions
[18- 20]. The spectra of both CuCl and CuBr were also investigated in detail
and new electronic transitions were found at a much later time [21, 22].
Intensity measurements on the visible bands of several
diatomic molecules were carried out and used in conjunction with the calculated
Morse Franck-Condon factors to estimate the variation of the electronic
transition moment with internuclear distance. These studies elucidated
contributions from other close lying electronic states to the electronic system
under investigation. RKRV potential curves were calculated using measured
spectral features and a comparison of these curves with empirical expressions
were utilized to estimate molecular dissociation energy [23]. These comparisons
enable one to draw qualitative conclusions regarding the nature of the
molecular bonding especially when ionic contributions are significant.
3.1
Vibrational Spectroscopy of Polyatomic Molecules & Potential Functions
Dr. I.S. Singh returned
from his post-doctoral researches in USA at the end of 1962 and the
investigations of infrared spectra of molecules were started using a
Perkin-Elmer 13U spectrophotometer. Assignments for the vibrational spectra of
tetracyclic hydrocarbons and their alkyl substituted derivatives were carried
out [24]. A large number of studies on the infrared spectra of benzene
derivatives were carried out and some of the representative studies in this
series consisted of work on o-, m-, p-dichloroanilines, o-bromotoluene and o-,
m-, p-fluoro and bromo- benzaldehudes [25- 27]. These investigations greatly
helped the analyses of electronic emission and absorption spectra of polyatomic
molecules by providing data from their vibrational spectra to assign vibronic
bands [28- 30]. Facilities were developed to grow single crystals of organic
molecules using the technique of zone-refining and polarized Raman and IR
spectra of single crystals of halogenated benzenes were investigated in detail
[31-33].
Around mid 1960s theoretical work on potential functions
and normal coordinate analysis was started to determine valence force constants
and mean square amplitudes in MXn type molecules using desk-top
calculators [34, 35]. Model force-fields, using different approximations, were
developed for these molecules and the general valence force constants were
calculated from additional experimental data e.g. frequencies from isotopic
molecules, Coriolis constants and centrifugal distortion constants. The force
constants for molecules with strongly coupled vibrations [36] and that for SF6
[37] are two such examples.
In the late 1960s, vibrational spectroscopic
investigations were supplemented with complete normal coordinate analysis on
digital computers of which the work on isobutene and isobutene-d8 is
an example [38]. In addition to the work on substituted benzenes [39-41], some
organometallics containing benzene ring were studied by Asthana and Pathak [42,
43]. A detailed study of the ground state potential surface of benzene was made
using vapour phase vibrational data on 14 different isotopic species by Thakur
et al. [44]. Excited electronic state force fields for styrene and
phenyl-acetylene have also been calculated [45]. Potential surface studies
based on the vibrational spectra of biomolecules- 2-thiocytosine and
8-azaguanine have also been carried out to understand some functional aspects
of such molecules [46, 47]. Dr R A Yadav and coworkers have studied vibrational
spectra and potential functions of a large group of molecules, including
biomolecules [48-54]
3.2
Electronic Spectra of Polyatomic Molecules
The availability of
10.6 m grating spectrograph since 1963 made it feasible to investigate the
electronic spectra of substituted benzene molecules under high resolution. The
rotational contours of electronic bands in the absorption spectra could be
recorded for parent as well as deuterated benzenes [55, 56]. The interpretation
of the rotational contours, in symmetric rotor approximation, in a group of halogenated
benzene molecules provided semi-quantitative estimates of change of molecular
geometry in going from ground to the excited electronic state [57, 58]. The
analysis of the rotational contours were later performed using computer
simulations and these were of great value in distinguishing between peaks due
to rotational transitions and those due to very small (≈ 1cm-1)
sequence intervals [59]. These studies also led to data on the orientation of
electronic transition moments in (ππ*)S1←S0 transitions
of substituted benzene molecules which in turn provide testing ground for
quantum mechanical calculations of these parameters.
Electronic
spectra of a number of aromatic molecules (benzaldehyde, benzoquinone and their
derivatives) characterized by one or more loosely bound electrons occupying
non-bonding molecular orbitals were extensively studied in the UV and visible
regions. Two systems of electronic bands observed in the vapour phase
absorption and emission spectra of these molecules were interpreted in terms of
a shorter wavelength π*←π electronic transition and the longer wavelength π*←n
electronic transition [60- 62]. The accidental coincidence of ground electronic
state C-O stretch frequency in benzaldehyde with the energy separation between
the triplet and singlet electronic states, due to π*←n electron promotion, had
led to incorrect assignments of the
extensive emission bands in benzaldehyde as well as in its halogenated
derivatives [63, 28]. The pioneering work of Stockburger on benzaldehyde at Schuler’s
institute in Germany led to the correct interpretation of the emission spectrum
in terms of a (nπ*)S1←S0 and a (nπ*)T1←S0
transitions. Subsequently the vapour phase emission spectra of benzaldehyde and
its halogenated derivatives were studied under high resolution to reveal the
two different types of rotational contours for the bands belonging to the S1←S0
and T1←S0 transitions [64-66]. These investigations
provided valuable information on the change of molecular geometry in going from
the ground to excited electronic states in halogenated benzaldehydes. The
vapour phase emission bands of the (nπ*)T1→S0 transition
in p-benzoquinone-h4 and –d4 were recorded and analyzed
for the first time in our laboratory [67] and were subsequently also studied
under high resolution [68, 69]. The studies on rotational contours of the
vibronically active bands of the (nπ*)S1←S0 transition in
pyrazine-h4,-d4 and -15N provide the first
example of switching of inertial axes in a molecule on electronic excitation.
In pyrazine the a-axis in S0 state, is along the two nitrogen atoms
and in the S1 state it is perpendicular to the line joining the two
nitrogen atoms [70]. This discovery generated considerable interest in the high
resolution spectroscopy of polyatomic molecules using lasers.
Naphthalene
is the simplest condensed ring hydrocarbon with D2h symmetry and it
has two specific non-identical sites referred to as 1 and 2 positions for
substitution of an atom or a group of atoms in place of the corresponding
hydrogen atom. Unlike the 260 nm benzene spectrum, the 310 nm absorption
spectrum, due to π*←π electronic transition, is electronically allowed in
naphthalene with the electronic transition moment along the long in-plane
symmetry axis of the molecule. In addition to the electronically allowed bands,
a group of much stronger vibrationally induced bands, with the transition
moment along the short in-plane axis, also appear in the absorption spectrum
[71]. The substitutions in naphthalene at positions 1 and 2 by an atom or a
chemical group affect the relative intensities of the electronically allowed
and vibrationally induced bands quite differently. Systematic studies on the
absorption spectra of substituted naphthalenes with halogen atoms, hydroxyl
group and amino group, have been carried out in our laboratory. The low
resolution investigations provided data on the electronic interaction between
the substituent and the carbon ring as changes of excited state energies [72,
73]. The High resolution studies on 1 and 2 substituted naphthalenes have led
to excited state rotational constants from which changes in molecular geometry
on electronic excitation have been determined [74]. Molecular orbital theory
has been used for a consistent interpretation of the electronic transition
moment orientation, in substituted naphthalenes, in terms of the chemical
nature as well as the site of the substituent [75].The electronic absorption
and emission spectra of a large number of naphthaquinones and anthraquinones
were studied in great detail to obtain experimental data on relative
intensities of the π*←π and π*←n transitions. A comparative study of the
excited state energies of these molecules was also made using semi-empirical
theories of molecular structure [76, 77].
3.3
Quantum Chemical Studies of Molecules
Dr D K Rai spent a year
during 1965-66 as a SIDA Fellow at the Kvant Kemiska Institution in Uppsala
University, Sweden in the group of Professor Per O. Lowdin. This visit was made
with the specific interest of using quantum mechanical theories to describe the
spectra and structure of molecules. On his return not only he initiated two
bright research scholars, P C Mishra and J.S. Yadav, into the new field but also
encouraged those involved in experimental investigations to make use of the
quantum chemical calculations in the interpretations of their findings. Since
substituted benzenes, experimentally studied in the laboratory, have one or
more of its atoms replaced by another atom or group, (e.g. F, Cl, Br, NH2,
OH, CH3 etc) it was natural to take up these systems for such
theoretical studies. The π-electron SC LCAO-MO theories in their semi-empirical
form were employed to describe the structure and spectra of the planar
conjugated systems.
For a planar conjugated system such as benzene it was
assumed that the 24 orbitals involved in σ-bonds provided a rigid framework in
which the 6 π-electrons in the delocalized bonds carried out their motion. The
near UV spectrum was attributed to excitation of one of these π-electrons from
the orbital occupied in the ground state to a more energetic orbital which was
empty in the ground state. The most popular method for such calculations was
the Pariser-Parr-Pople (PPP) method in which a number of integrals were
approximated either by data taken from experiments or from other empirical
considerations. A modified PPP procedure known as iterative PPP was devised
where, after each SCF cycle a new set of values for the parameters was
evaluated and the process continued till no further changes were necessitated
[78, 79].
Experimental
studies, to quantitatively measure the changes in molecular geometry for
substituted benzenes on electronic excitation, are extremely complex because
the three moments of inertia, (obtained from rotational analysis) are not
sufficient to fix all the geometrical parameters. Estimates of changes in C-X
bond length (where X is the substituent) could be obtained by making certain
plausible assumptions. It was, however, impossible to choose between two
equally probable values. It was therefore, thought wise to use a theoretical
procedure that would make the choice unambiguous. Since the SC procedure yields
the bond order matrix for ground state, the CI wavefunctions could be used to
obtain the bond order matrix for the excited state by assuming a probable
assignment for the electronic transition. It was further assumed that the bond
order- bond length relationship suggested by Coulson, for conjugated systems,
can be used to estimate bond lengths in the excited state. It was found that
even the simple π-electron study yielded reasonable estimates [80]. Application
of Coulson’s relation, required the evaluation of the σ- and the π-bond orders
separately, and it could not be applied directly to the bond order matrix in
SCF calculations where all valence electrons were included. A procedure for
transforming the density matrix to enable separation of the σ- and the π-bond orders
was, therefore worked out [81]. These σ- and the π-bond orders were used in
Coulson’s relation to successfully estimate the bond length changes [82, 83].The
application of quantum chemical techniques has been successful in
conformational studies of biomolecules also [84-86]. In all cases it is the
conformational structure of the di- and polysaccharides which are of interest.
Both semiempirical CNDO type methods, including PCILO and molecular mechanics
methods, have been used. The studies started with monosaccharides, Glycan
moiety and N-Acetylglucosamine and progressed through di-acetyl glucosamine and
other dimmers to tri, tetra and pentamers of some units.
There
were no computing facilities on the BHU campus in those days and the research
scholars had to travel to either Indian Institute of Technology (IIT) at Kanpur
to work on an IBM 1620 Computer or to Tata Institute of Fundamental Research
(TIFR) at Bombay which had a CDC 3600 Computer. Professor P Venkateswarlu at
Kanpur and Dr N A Narasimham at Bombay were to be approached in case of any difficulties
and they most willingly provided the necessary help to the students from their
alma mater.
3.4
The Ruby Laser in Spectroscopy Laboratory
Professor N L Singh had
invited Professor J G Winans, of the State University of New York at Buffalo in
USA, to come to the Spectroscopy laboratory of BHU for the academic session of
1968. Dr R B Singh had gone to SUNY at Buffalo for a year and Professor Winans
was so impressed with his expertise that he came to BHU with a large crate
containing the power supply and the bank of heavy condensers for the
flash-excitation of Ruby laser. The detection of the laser fluorescence and
laser Raman scattering was photographic of a type that did not exist in our
laboratory. It is known as Polaroid photography.
Our method of photographic detection consisted of manually developing the
exposed films and plates in the dark room containing developer and fixer
solutions. The film pack that Professor Winans had brought with him was
semi-automatic and very fast. After exposure one could activate the chemical
processing of the film in the pack itself so that within a few minutes when the
covers were removed the fully developed film would emerge from the pack.
Although there was no major research carried out with this system, we learnt a
great deal from the demonstration experiments carried out in the laboratory.
Professor Winans also gave a series of lectures on the theory and application
of lasers. Thus within a decade of its discovery we could visualize the impact
of laser as source of monochromatic light in the science of spectroscopy.
4.
Lasers, Lasers Spectroscopies, Atomic & Molecular Collisions, Biomolecules
(1975-95)
The spectroscopy
department was merged into the parent Physics Department in 1971 and the
academic atmosphere became very congenial with great deal of cooperation among
the teaching staff and a healthy competition to excel in the various areas of
research. Professor B A P Tantry was the senior most professor in the field of
electronics, Professor G S Verma in theoretical condensed matter physics,
Professor P Krishna in experimental solid state physics, Professor P C Sood in
nuclear physics and Professor D K Rai in atomic and molecular spectroscopy. Professor
Rai was the youngest and full of many ideas of diversifying the researches on
atoms and molecules. His grasp of the intricacies of quantum mechanics was of a
very high level and his understanding of the experimental intricacies of high
resolution spectroscopy had acquired great deal of maturity during a decade of
working with experimentalists like Prof. N L Singh, Dr I S Singh, Dr K N
Upadhya and Dr S K Tiwari. He exhibited immense patience in talking to junior
members like us and greatly appreciated if we had any new ideas in the field of
experimental investigations or theoretical problems. There was a perceptible
change in the research atmosphere of spectroscopy which had so far, been
lacking, to some extent, in the tools of theoretical inquiry.
Professor D.K. Rai (1943- 2012)
I was very fascinated by a talk by Professor C A Coulson
of Oxford University entitled ‘Shape of molecules in excited electronic states’
at the 1967 International Conference in Spectroscopy at Bombay and I had put
his Institute as one of the places to go for higher studies in my application
for a 1851 Exhibition scholarship of the Royal Commission of London. After my
final selection Professor Coulson advised me to join the Spectroscopy group in
the Chemistry Department of Reading University headed by Professor I M Mills.
During my two and half year stay I worked on high resolution electronic
spectroscopy of polyatomic molecules and made use of digital computers for
analyzing the rotational contours of electronic and vibronic bands. I used to
frequently visit the group of Professor Coulson at Oxford which was involved in
theoretical aspects of molecular structure and molecular dynamics and I also
attended one of his famous Summer Schools. In the physics department at Reading
University I listened to a fascinating talk by Professor G W Series on hydrogen
atom. Professor A W Schawlow was also in the physics department for a brief
period working on a dye laser and he presented a popular talk on Lasers in
which he exhibited the properties of transmission and absorption of a laser
beam from his toy laser gun that included a real ruby laser inside. He had a
double balloon- a clear shell with a dark blue one inside. The pulsed laser
beam blasted the inner balloon while leaving the clear one intact. This showed
that laser could pass through the transparent balloon without burning it.
Towards the end of my stay in England I visited the spectroscopy laboratory of
Dr M Stockburger in Gottingen, Germany where I witnessed the proto type of
Nitrogen laser pumped dye laser system being developed by Dr F P Schafer. On my return to BHU in 1974
I talked to Professor Rai about the feasibility of doing laser spectroscopy and
we came to the two pronged plan of building some simple laser systems and apply
for funding to purchase the expensive commercial lasers for state of- the- art
laser spectroscopy.
The period from 1975 to
1995 was one of the most productive interval during which a great deal of
diversification in teaching and research in spectroscopy was made. Professor
Asundi visited the laboratory one last time in 1978 when, one of his most
favourite first batch students, Professor Jagdeo Singh also happened to be in
BHU and we have a rare group photograph of three generations of teachers and
students of the Spectroscopy laboratory of BHU. With the addition of Lasers in
the spectroscopy group it was decided to rename it as Laser & Spectroscopy
Laboratory. It seems pertinent to me to quote, again, from some of the messages
received on the occasion of the 50th anniversary.
Professor P M Johnson of SUNY at Stony Brook and
Brookhaven National laboratory wrote, “On the occasion of thr fiftieth
anniversary of the Banaras Hindu University Spectroscopy Laboratory, I wish to
extend my warmest appreciation of the considerable past and ongoing
contributions made in your laboratory. From the pioneering work of R K Asundi
to the present, scientists working in Varanasi have unraveled the intricacies
of molecular spectra with talent and perseverance which must stem from essence
of Hindu tradition. During my visit last year I was impressed by the dedication
of your department in not only carrying out research, but of furthering science
in India through the organization of conferences and workshops. With this kind
of effort the next fifty years should prove to be as productive as the last.”
Professor Alexander Dalgarno of Harvard-Smithsonian
Center of Astrophysics wrote, “I remember with great pleasure my visit to your
famous laboratory in December 1983, where I was able to observe that the
tradition and creative research initiated by your distinguished predecessors
was being maintained and extended in a rapidly changing field of endeavor. I
was greatly stimulated by the atmosphere of intellectual inquiry and by my
personal interactions and discussions with you.”
Professor S Ramasesan of Raman Research Institute wrote,
“The laboratory which was founded by the illustrious spectroscopist Prof.
Asundi is today one of the best spectroscopy laboratories in India. I
congratulate its Alumni on their achievements and greet the laboratory on this
auspicious occasion.”
4.1
The Home-built Lasers
Some very enthusiastic
research scholars, J P Singh, V N Rai and A K Rai among them, with expertise in
electronics joined the Laser and Spectroscopy group with interest in
fabricating lasers and doing research in laser spectroscopy. They were ably
assisted by an electronics engineer Mr Ramji Rai and by the end of 1978 it was
possible to make several working units of low pressure as well as high pressure
N2 lasers emitting in the near UV region and flashlamp pumped planar
dye lasers emitting in the visible region. Parametric investigations on the mode
of working of these laser systems were carried out and the laser emissions were
photographically recorded to reveal spectral characteristics under different
conditions of excitation. These investigations resulted in a number of research
publications [87- 91]. Later dye laser systems pumped by N2 laser
emission at 337.1nm were indigenously fabricated and used in investigations on
excitation transfer in dye mixtures [92].
4.2
Laser Spectroscopy of Atoms and Molecules
The Physics Department
received its first major research grant under the Special Assistance Program of
UGC, New Delhi after the recommendations of an expert committee. Professor S
Ramsesan of I.I.Sc., Bangalore, on the committee, was very impressed with the
two pronged approach of fabricating lasers and using commercial lasers for the
state of the art laser spectroscopy and it was on the basis of his strong
recommendation that a 4W Spectra Physics Argon laser was procured by the
spectroscopy laboratory. A linear dye laser pumped by the Argon laser was also
later added to carry out laser fluorescence and other tunable laser based
spectroscopic research. Laser fluorescence investigations on CuI molecule led
to re-examination of the earlier assignments, using conventional spectroscopy,
and it was found that the earlier assignment was correct [93, 94]. The
technique of polarization labeling was used to explore high energy Rydberg
states of Li2 leading to some very interesting results [95].
Collision induced fluorescence from triplet states of Li2 have also
been observed which provide spectroscopic information on triplet states [96].
The effects of laser light on germination of seeds and growth of seedlings were
investigated with and without exposures to radiation from the Argon laser [97]
Laser
induced two-photon spectroscopy of polyatomic molecules was carried out using
the technique of multi-photon-ionization detection in a vapour cell [98, 99] as
well as in a supersonic molecular beam [100]. Studies on two-photon spectra of
aniline [101] and dichlorobenzene [102, 103] along with three-photon spectra of
diatomic molecules were carried out [104].
4.2.1
Laser Photoacoustic Spectroscopy
In the spring of 1977,
I went to attend an International Workshop on Lasers at ICTP in Trieste, Italy.
I was invited by Dr Hollas for 4 weeks in Reading University before spending
some time at the National Institute of Optics with Professor Tito Arecchi in
Florescence, Italy. Towards the end of my stay in Reading, Dr Hollas took me to
Bristol for a one day meeting on spectroscopy. Christopher Webster was a Ph D. scholar of
Professor R N Dixon, and he showed his photoacoustic detection system to me
with great enthusiasm. This photoacoustic spectrometer was novel in the sense
that it detected the pressure signals generated by periodic heating produced by
absorption of light of different wavelengths from a tunable laser source. The
concept was based on the experiments carried out by Graham Bell in USA about a
century back in his attempt to transmit sound over a beam of light and who
invented the ‘spectrophone’ by modifying a spectrometer where the eyepiece was
replaced with a hearing tube (Fig.4).
When I talked to
Professor Arecchi, on my arrival in Florence, about this new kind of
spectroscopy, he asked me to give a short presentation for the members of his
group working on various applications of lasers. I made a high pressure N2
laser during my stay in INO at Florence and visited the Florence University to
see the variety of researches on flashlamp pumped dye lasers in the group of
Professor P Burlamacchi and also met Professor S Califano working in the field
of vibrational spectroscopy whom I had known, while working on molecular force
constants, from India. This short visit turned out to be very profitable
because Professor Arecchi had advised me to get in correspondence with Dr M B Robin,
in the field of photoacoustic spectroscopy, at the Bell Laboratory in USA.
Dr Robin, sent me, at the BHU address,
not only several of his recently published papers in photoacoustic spectroscopy
but also a few square meters of metal coated Mylar film to make the sensitive
microphone. With the help of our very experienced and capable mechanic Mr.
Santlal, the enthusiastic group of research scholars, J P Singh, V N Rai and L
B Tiwari, got the electret microphone made and tested it in the photoacoustic cell,
using a 0.25 m grating monochromator and a white light source.
Fig.4 The Spectrophone of Graham Bell with a Hearing Tube
A parametric study of
the photoacoustic signals generated by Argon laser was carried out to develop
the design of the photoacoustic cell for measurements on solid and liquid
samples in our laboratory [105] exactly hundred years after the original
discovery of the ‘Photoacoustic Effect’ by Graham Bell [106]. Our photoacoustic
spectrometer has since been used for a variety of experiments on samples in the
form of powders, thin films and solutions. Photoacoustic measurements of CuSO4 powders, as a function of temperature, have led to
detection of phase transitions as a result of structural changes in the
molecules [107]. The effect of neutron irradiation on rare earth oxide powders
has been monitored by measuring the photoacoustic signals at regular intervals
of time after irradiation and the results indicate a change in the oxidation state
of the rare earth atoms [108]. The nonradiative transitions in dye solutions
are a major cause of lowered lasing efficiency in dye lasers. Measurements of
photoacoustic spectra of dye solutions, of different concentrations and of
mixtures of two dyes, were carried out to provide data that may be used for
optimized lasing efficiency [109]. Photoacoustic measurements on carbon black
led to the development of a simple laser power meter [110] and chaotic behavior
of photoacoustic signal was investigated as a function of laser power [111].
Studies on photoacoustic spectra of Calibo glass samples doped with rare earth
ions were also carried out to understand the mechanism of nonradiative transitions
in these systems [112]
Pulsed laser generated
PA signals from powder samples have been recorded with the experimental
arrangement shown in Fig. 5 and a home-made piezoelectric transducer whose
details are shown on the right of the experimental setup [113, 114]. A thin
circular layer of the powder sample was sandwiched between two plane glass
plates and the PZT transducer was tightly held with its front face in firm
contact with one of the glass plates. The linear separation between the sample
spot and the face of the transducer was earlier determined by maximizing the PA
signal, while recording the spectral profile of the dye laser output, by using
carbon black in place of the sample. The wavelength of the dye laser output was
calibrated using the opto-galvanic signals generated from a Fe-Ne hollow
cathode lamp. The powder sample consists of micro-crystals of Ho2O3
and the light absorbing species are Ho3+ ions in these
micro-crystals. The acoustic pressure wave generated in the glass plate,
following the pulsed optical absorption of the sample, propagates to the
transducer where these are converted into transient electrical signals as a
result of the piezoelectric effect. The transient PA signals are then processed
by the boxcar average and the spectrum recorded by tuning over the emission
wavelengths of the dye laser.
Fig.5 The set up for PA spectroscopy with tunable dye laser (left) using a piezoelectric device (right)
Fig.5 The set up for PA spectroscopy with tunable dye laser (left) using a piezoelectric device (right)

|
The photoacoustic
signal from gaseous samples is greatly affected by the shape and size of the
cell as well as the location of the microphone inside the cell. Parametric
design of photoacoustic cells with longitudinal resonance at the chopping
frequency for a continuous laser showed that maximum signal is registered by
the microphone when it is located near an antinode (see Fig. 7) and the signal
decreases to a minimum if positioned near a node [116]. Pulsed lasers as well
as periodically chopped continuous lasers have been used for measurements of
one and two-photon absorptions in gases and molecular vapours [117- 119].\
Fig.7 Schematic diagram of a PA spectrometer for
gaseous sample. The cw CO2 lasr is attenuated down to a few
milliwatt output power before passing through the mechanical chopper
4.2.2
Laser Photothermal Lensing Spectroscopy
Photo-thermal lensing
effect also depends on the non-radiative characteristics of fluid samples when
irradiated with laser light (called pump laser). The heat produced is maximum
at the absorption wavelength of the sample and it leads to a decrease of refractive
index. If a second laser beam (called probe laser) with no absorption by the
sample passes through the medium in the presence of the pump laser light then
the former is deflected by an amount proportional to the heating produced by
the latter. The detection of the probe beam (of fixed wavelength) intensity, as
a function of the wavelength of the tunable pump laser, is called photo-thermal
lensing spectroscopy. A dye laser pump and Helium probe laser set up was used
to study the overtone bands due to C-H stretching vibration in a number of
substituted benzenes [120- 126]. A similar set up was used for recording the
photoacoustic spectrum of Br2 vapour which was found too corrosive
to use the microphone detector [127]
4.2.3 The Joshi Effect and Laser Optogalvanic
Spectroscopy
Professor S S Joshi of
the Chemistry Department was the Principal of Science College and he played a
major role in bringing Professor Asundi to BHU. Prof. Joshi observed fluctuations in the
discharge current, when irradiated by external light, for a variety of atomic
and molecular vapours in the discharge tube [128- 130]. His experimental set up
is shown in Fig. 8. The inner electrode of the discharge tube is to be
connected to the positive polarity of a stabilized high voltage power supply
and the outer electrode is connected to ground. α, β and
γ represent three different electric
circuits for the detection by a counter, a galvanometer and an oscilloscope
respectively. For the latter two methods of detection the electric supply was
from a low frequency transformer and the current passed through a Sylvania IN34
rectifier. The shape of the signal on the oscilloscope was controlled by
adjusting a carbon resistor. The change in the magnitude of the discharge
current constituted the signal and this phenomenon was termed as the Joshi
effect and was explained in terms of the change of rate of ionization following
light absorption. Several research scholars, of the first batch working in
spectroscopy laboratory, carried out experiments involving this optically perturbed
discharge phenomenon [131]. In 1928 Penning [132]
had also observed that a change in the breakdown voltage of an electric
discharge cell containing a mixture of neon and argon occurs when it is
irradiated with light from another discharge cell containing only neon gas and
explained it in terms of Penning ionization. This phenomenon is now popularly
known as the Opto-Galvanic effect.
The opto-galvanic
effect corresponds to an increase or decrease in the discharge current
depending upon the kinetics of the population and depletion of the levels which
are perturbed by the laser radiation. In the case where laser excites atoms
from a level with smaller net rate of ionization to a level having a larger
rate of ionization, the conductivity of discharge increases leading to increase
in discharge current. In the reverse case, laser transfers atoms to a level
with a smaller rate of ionization, leading to a decrease in the discharge
current. The rate of ionization of atoms depends on the excitation energy and
the lifetime of the excited state. The steady discharge current I0
exhibits a temporal variation when a short laser pulse (≤ 10-8 sec) from a tunable
laser, resonant with one of the emission
lines of discharge, hits the region of the electrical discharge. The deviation
from the steady current may persist for 10 to 100 µs after the incident light
pulse and it is given by I(t)
= I0e(K-1)t/τ where K is a multiplication factor and depends on
the two energy levels of the atom (or molecule) responsible for the emission
line. τ
is the mean time of one generation of electrons in the multiplication process
initiated by the laser pulse.
Fig.8 Experimental arrangement for
recording Joshi effect
The changes in discharge current in
the opto-galvanic effect are very small and require a phase sensitive
electronic detection system. A typical experimental set up is shown in Fig. 9.
The laser optogalvanic spectrum of discharge through I2
vapour was studied in the wavelength range of 530- 630 nm corresponding to the
B-X system of electronic bands. Variation of the external voltage across the
discharge tube was found to lead to very significant changes in the
optogalvanic signals corresponding to different bands in this region. The
results have been interpreted in terms of magnetic predissociation of I2
molecule [133]. Similar observations were made in other halogen and inter-halogen
molecules [134, 135]. The D.C. discharge through HgBr vapour generates intense
bands in the blue-green region of its visible spectrum. An optogalvanic
measurement of HgBr discharge using Argon laser was also explored [136] and
two-photon optogalvanic measurements on C-X and D-X transitions in this molecule
were later carried out [137, 138]. Optogalvanic spectroscopy of neon discharge
involving one and two-photon transitions including a convergent Rydberg series
(see Fig.10) have provided information on transitions originating in metastable
excited states [139, 140]. A detailed account of laser optogalvanic studies at
BHU along with those in other laboratories has also been published [141]
Fig.10 The ‘ns’ and ‘nd’ odd
Rydberg series in 2-photon optogalvanic spectrum of Ne originating in 3s[3/2]02
metastable state (using wavenumber scale)
4.2.4
Laser Raman Spectroscopy
Dr S B Rai and Dr B P
Asthana were spectroscopy students from the last batch of its existence as a
separate department. Both of them were selected as Alexander von Humboldt Fellows in late 1970s to work in
Germany. Both of them later became professors in the department of physics, the
area of research of Dr Rai has been mostly electronic spectroscopy, that of Dr
Asthana was vibrational spectroscopy and both of them have brought much glory
to the Laser and Spectroscopy Laboratory. The tragic death of Professor B P
Asthana in 2012 has been a tremendous loss to Raman spectroscopy in general and
to BHU in particular.
Professor B.P. Asthana (1952- 2012)
The heavy cost of
importing complete instrumentation, for carrying out experimental work in Laser
Raman Spectroscopy, did not make it possible to install experimental facilities
in this field till late 1980s. Dr Asthana went to the famous Raman spectroscopy
laboratory of Prof. W Kiefer in early 1980s and carried out cutting edge
research in this very important field. He was so well recognized for his
expertise, both theoretical and experimental, that he became almost a member of
Prof. Kiefer’s famous group. He took this opportunity to bring the fruits of
his efforts to BHU by visiting Germany during the summer vacations, carrying
out research there, to bring back a vast amount of experimental data to be
analyzed by his students in BHU. He had tremendous capacity of working long
hours without any break, even from his student days, which made it possible to
indirectly equip BHU in the field of Raman spectroscopy. His
early researches involved deconvolution of Lorentzian linewidth from observed
Raman lines, precise frequency shifts by Raman difference spectroscopy and
studies on hydrogen bonded molecules [142- 146]
A chapter on ‘Vibrational line
profiles and frequency shift by Raman spectroscopy’ was written in the advances
in Vibrational Spectra & Structure [147] and a series of papers were published
on vibrational dephasing, on, application of density function theory for
optimization of molecular geometry and vibrational spectra, and on femtosecond
resolved Coherent Antistokes Raman Spectroscopy (CARS) [148-150]. Temperature
dependent Raman studies in liquid crystalline systems [151, 152], in isotopic
dilution and self association [153] and in concentration dependent vibrational
shifts [154, 155], were supplemented by ab initio calculations. An ab initio
simulation study of benzene Raman intensities has also been carried out [156]. Studies on Raman scattering in magnetic
materials and polarized Raman spectroscopy have been carried out by Professor R
A Yadav [157, 158]
4.3
Atomic & Molecular Collisions
Electron impact
processes make an important contribution to the gaseous discharges, through
atomic and molecular vapours and gases, which have been used as the most common
sources for emission spectroscopy. The collisions between various charged and
neutral species, present in the laboratory or atmospheric discharges, is an
area of great significance for understanding the mechanism of excitation and
ionization. The studies of collision phenomena leading to calculations of the
relevant cross-sections started around 1970 and many research scholars worked
for their Ph.D. degree in this area. A systematic theoretical analysis of the
processes of direct ionization, innershell vacancy production, electron
capture, double ionization and energy loss of energetic projectiles etc were
made by Prof. D K Rai and his students. Dr. R Shanker started
developing the experimental facilities for atomic- and molecular collision in
mid 1980s after he joined the faculty with his many years of experience in
Canada and Germany. Initial studies were made on electron bremsstrahlung
spectra emitted from thick solid targets with impact of electrons obtained from
an indigenously built 1-8kV electron gun.
The theoretical studies were started along the lines of
Gryzinsky’s application of the classical theory to the problems of atomic
collision physics. Gryzinski had prescribed algorithms, during the mid 1960s,
for calculation of electron (as well as heavier charged particle) impact single
and double ionization of atoms and molecules. Double ionization and excitation
was presumed to proceed via two independent mechanisms. In the first mechanism
the two ionized electrons resulted from two successive collisions of the target
atom with the incident particle, while in the second mechanism one of the two
ejected electrons came due to the electron which was ejected by the incident
particle. The initial studies in our laboratory were involved with the test of
the various prescriptions given by Gryzenski in a variety of cases and the
importance of using a correct target electron velocity distribution was
emphasized [159, 160].
Gryzenski had suggested that an incident proton could
capture an electron from the target atom if the energy transfer lies between
two specified limits which depend on the binding energy of the proton-electron
system. Other workers, however, were of the view that electron capture ought to
be described as a result of two separate binary collisions – the first
collision between the incident proton and the target electron which frees the
latter from the field of the target nucleus (ionization) while the second
collision takes place between the ejected electron and the nucleus and causes
the former to move parallel to the incident proton with a speed suitable for
capture. The idea of double collision and a geometrical model of the atom were
used to derive improved limits for energy transfer as necessary for electron
capture. The numerical results obtained in this manner were much better than
those with the Gryzenski limits [161, 162].
The problems of direct inner shell ionization and
autoionization of an innershell electron were solved by using Born approximation
[163] and also more improved approximations [164, 165]. These calculations use
an effective complex charge whose consequences for the calculated cross sections
have also been examined [166]. A new procedure for calculating the third term
of the Glauber series has been suggested and applied for e- H scattering [167].
In a different study a Hylleraas type correlated wavefunction has been used to
calculate double excitation in He [168].
The first experimental set up was used with the 400 kV
Van de Graaff accelerator as the projectile machine for ion-atom collision
experiments. The study of non-uniform population of the magnetic substates of a
vacancy created in ion-atom collisions, with the total angular momentum J
greater than ½ was undertaken [169]. The inhomogeneous population of various
magnetic substates with respect to the incoming ion-beam is normally referred
to as ‘alignment’ parameter. The innershell vacancies of heavy Z-elements with
J= ½ are investigated for this parameter in the range of kilovolt projectile
energies. The projectiles available from the Van de Graaff accelerator were
protons, deuterons and He. Measurements of the angular distribution of the
X-rays emitted due to the decay of innershell vacancies yield the desired
parameter (‘alignment’) as a function of the projectile energy. The scattering
chamber fabricated in our workshop facilitated the photon detector [Si(Li)] to
be rotated about the collision center and thin films, either self supported or
carbon backed, were chosen as targets [170, 171].
4.4
Spectroscopy and Quantum Chemical Computation of Biomolecules
The nucleic acid bases,
their analogues and derivatives are of great importance in the understanding of
processes in biology and medicine such as in photobiology, mutation and cancer
research. Electronic spectra of nucleic bases were investigated and it was
found that semi-empirical SCF-MO-CI theory could explain the spectra of
pyrimidines reasonably well but for purines it was not so successful [172-175].
Studies on photo-dimerisation and photo-hydration of pyrimidines showed that
C5C6 bond order is strongly reduced and free valences at C5 and C6 sites are
enhanced in uracil and thymine in going to lowest singlet and the lowest
triplet excited states in a π*←π excitation. This is in agreement with the
experimental observation that photodimerisation occurs at the C5C6 bond in
these molecules. The electronic spectra of guanine, adenine and some purines
have been explained in terms of asymmetric double well potential surfaces for
their ground as well as the excited states [176-182]
In the studies on processes of Phototautomerism and Photoisomerisation of
biologically important molecules, 2-aminopurine revealed a high quantum yield
with lifetime of the excited state in nanosecond range [183] while fluorescence
from urocanic acid was observed for the first time [184]. A number of studies,
employing quantum chemical methods, were undertaken on the mapping of
electrostatic potentials and electric fields of biomolecules and these were
correlated with their hydrogen bonding abilities which provide important
information on drug design [185- 187]. Several calculations were carried out to
elucidate the biological processes in Adenine and Guanine [188- 191]. DFT study
has been made on the protection against radiation induced damage in DNA [192]
and theoretical studies on reactions involved in the formation, of
8-Nitroguanine [193] and of Ferulic acid and Vanilin [194], have also been
carried out.
5.
Laser Spectroscopy of Materials, Nano-materials, Particle-impact Spectroscopy, LIBS
(1995- 2014)
The spectroscopy
laboratory was recognized as a National Center for Laser Spectroscopy and an
Argon laser pumped Ti-sapphire and dye laser was sanctioned by the Department
of Science and Technology, New Delhi in early 1990s. The availability of laser
radiation in the near IR provided opportunities to excite low energy electronic
states of atoms molecules and ions to carry out spectroscopic investigation on
such systems in addition to those which could be explored with visible light
from the Argon laser or the dye laser. The Physics department had been
recognized as a Center of Advanced Studies by the University Grants Commission
with Studies of Materials as the thrust and this provided enough incentive to
focus the research work on condensed materials. Glasses have some unique
properties e.g. transparency and high hardness at room temperature along with
their strength and excellent resistance to corrosion. Glasses have potential
applications in optical fibers, lasers and in many areas of engineering and
technology. Prof. S B Rai and his
research scholars have synthesized and investigated a variety of rare
earth-doped glasses, ceramics and nano-materials that have many applications in
the field of sensors in view of their novel spectroscopic characteristics.
There has been a large research output from the laboratory on studies involving
Glassy materials, Ceramic materials, Nanoparticles and Biological materials. In
the following sections a brief description of the research activities from mid
1990s till 2014 has been summarized.
5.1
Laser Spectroscopy of Glassy Materials
Oxyfluoroborate glass
matrix has been extensively used in the spectroscopic investigations of rare
earth (RE) ions. Fluorides are known to have a large capacity for RE metals and
offer a higher RE ion luminescence efficiency in comparison with oxides. At the
same time the glass ceramics possess high mechanical and chemical stability
characteristic of oxides. They also possess functional properties typical of
crystallites embedded in a glass matrix and have technological advantages
inherent in the glass preparation process. Spectroscopic properties of
oxyfluoroborate glasses doped with RE ions Tb3+, Gd3+, Nd3+,
Ho3+, Er3+, Eu3+ and Dy3+ have been
investigated in great detail [195- 201]. Effect of thermal neutron impact on
the spectroscopic properties of Gd3+ doped oxyfluoroborate glass
[202] and the optical properties Eu3+ from nanocrystallites in
oxyfluoroborate matrix [203] were also investigated. Borate glass matrix has
been used for the investigations on the spectral features associated with Dy3+,
Sm3+ and Pr3+ [204-206]. Cerium doped oxyfluoroborate
glass has strong absorption in the ultraviolet and red shift of the absorption
as well as emission bands has been observed with increasing cerium
concentration. Two different emission centers of Ce3+ have been
found in the glass matrix [207]
Heavy metal oxide glasses modified with RE elements have
attracted much interest in the past decade with the aim to shift the absorption
edge to mean IR region for applications in laser glasses and fiber optics in
this region. Tellurite glass combines the attributes of wide transmission
regions and good corrosion resistance, low phonon energy, high refractive index
and very good RE ion solubility. Spectroscopic characteristics, of Eu3+,
Sm3+, Ho3+ and Tb3+ doped in tellurite glass
matrix, have been investigated to find their possible applications in optical
technology [208-211] and a temperature dependent study of Pr3+ doped
sample has also been carried out [212]. The mechanism of optical frequency
upconversion in single RE ion doped tellurite glass matrix [213- 218] as well
as zinc phosphate glass [219] has also been studied. Energy transfers from Yb3+,
to Eu3+ co-doped in oxyfluoroborate glass (220) and to Tm3+
in lithium modified tellurite glass [221] give rise to upcoversion of 980 nm
laser radiation into visible light in the red and blue regions.
The investigations on upconversion
phenomenon are motivated not only by attempts to understand the mechanism of interaction
between the rare earth ions doped into a variety of host materials but also the
search for new light sources emitting in the visible region. It thus becomes
necessary to select solid host materials doped with rare earth ions that show
strong and broad absorption bands matching with the emission wavelength of
commercial lasers. The process of upconversion depends on the composition of
the glass matrix and may involve multistep absorption in a single RE ion or it
may result from energy transfer between groups of RE ions. This is illustrated
by the studies on Er3+ doped in BiLiBaPb glass [222] and in
tellurite glass in presence of Yb3+ [223]. Nanosize Pr:Y2O3
crystals have exhibited two-photon upconversion at room temperature when
excited by 532 nm laser [224] and Tm3+/Yb3+ co-doped Y2O3
nanophosphers emit intense light at 478 nm when excited at 976 nm [225],
whereas Er3+/Yb3+ co-doped Y2Te4O11
nanocrystals exhibit multicolour down and upconversion when excited at 266 and
976 nm [226]
5.2 Studies on Exotic Nano-materials
Graphene
is a novel nanomaterial which consists of one atom thick layer of carbon atoms
in a hexagonal crystal lattice. The peculiar dynamic complex conductivity of
grapheme enables the propagation of tightly confined electromagnetic modes at
the interface between grapheme and a dielectric material. These are commonly
referred to as surface Plasmon polariton (SPP). The propagation of SPP waves is
supported by many metals and metamaterials, but at very high frequencies in IR
and optical bands. However SPP waves on grapheme have been observed at lower
frequencies in the terahertz band and these can be tuned by material doping.
This type of behavior has stimulated many kinds of theoretical investigations.
Professor P C Misra and his research scholars have employed the methods of
density functional theory to illustrate the properties of grapheme using their
finite size models in the form of polycyclic aromatic hydrocarbons and their
poly radicals. These systems have the property of enhanced electron density at
their edges which explains the observed pronounced edge reactivities. It has
been found that the enhanced edge electron density continues to occur in dimers
and trimers of these model systems as well as in their excited states [227, 228].
Professor
R A Yadav and his research scholars are interested in structure and dynamics of
organic molecules with exotic characteristics, photorefractive materials with
non-linear optical properties and electromagnetic functioning of optical
fibers. For nucleic acid bases, molecules of medicinal importance and organic
molecules exhibiting conduction and superconduction properties, the vibrational
spectra are experimentally recorded to study their behavior. The analyses of
these spectra are performed using ab initio and DFT computation by employing
the Gaussian software [229-235]. Maxwell’s equations have been employed in the studies on spherical
dielectric resonators with metallic shields that protect energy leaking out of
the resonators [236. 237]. Photorefractive materials provide an ideal
medium for non-linear interaction of two or more electromagnetic waves with
many important applications. A number of theoretical studies have been carried
out involving resonators that may facilitate interactions in photorefractive
media [238- 241].
Professor
S B Rai, Dr Amresh Bahadur and their co-workers have carried out experimental
spectroscopic investigations on a variety of synthesized glassy, crystalline
and nanomaterials. These novel materials have important applications as
temperature sensors, as optical nano heaters, as fingerprint detectors and as
devices for bioimaging [242-250]. Indian traditional medicine ‘Ayurveda’ makes
use of unique metallic-herbal preparations called ‘Bhasma’. This involves many
processing steps that may include repeated calcinations of metal in presence of
natural precursor e.g. herbal juices, decoctions and powders. It has been
recently found that “Bhasma’ contains sub-micron size or nano particles and
different nutrient elements. Systematic spectroscopic studies have been carried
out to understand the role of natural precursors in detail [251-254].
5.3 Particle
Impact Spectroscopy of Atoms and Molecules
Prof R
Shanker and his research scholars have developed sophisticated experimental
facilities, with generous funding from various agencies, for investigations on
collisions of atoms and molecules with electrons, protons and ions which are at
par with international research in this field. The
first modern functional experimental setup [Fig. 11] was developed in 1996 to
study the X-ray, Auger- and recoil ion spectroscopy of thin (gas) and thick
(solid) targets under INSA-DFG international research program. Collisions of keV
electrons with atoms and molecules has made it possible to obtain the shape of
bremsstrahlung photon energy spectra and investigate thick targets [255-258].
Fig.11. Photograph of
experimental set up for use with Van de Graaf accelerator
Two
electron processes in the ionization of molecules by proton impact were
investigated [259] and the setup for coincidences between recoil and projectile
ion (COPRPION) facility at Nuclear Science Center (present IUAC) in New Delhi
was developed for coincidences in energetic heavy ion-atom collisions [260].
Characteristic and non-characteristic X-ray emission from molecules under
electron impact have been studied [261] and cross sections as well as
efficiency of bremsstrahlung under electron impact below as well as above 6 keV
have been investigated [262-264]. Multiple ionization of argon in coincidence
with projectile ions in 60-120 MeV range were investigated [265] and a time of
flight spectrometer was fabricated for studies on multiple ionization of gases
by charged particle impact [266]. An experimental set up for studying
collisions of keV electrons with thin and thick targets was also designed and
built [267]. Ejected electron –ion coincidence measurements were carried out in
multiple ionization of argon [268] and in dissociative ionization of SF6
[269] by electron impact. Energy and angular momentum distribution, of
electrons ejected from molecules during electron impact experiments, have been
measured in the laboratory [270]. Measurements have been made on the mean
transverse kinetic energy of recoil ion and on differential partial ionization
cross section of atoms during electron atom collisions [271, 272]. Measurements
on energy and angular distributions of backscattered electrons from tungsten target
have been carried out [273- 276]. Studies on bremsstrahlung spectra produced
from thick targets under kilovolt electron impact experiments led to some very
interesting results [277, 278]. Electron-molecule impact experiments have
produced data on partial ionization cross sections and dissociative ionization
cross sections for CO2 molecule [279, 280]. Measurements on
electron-molecule impact
experiments have produced valuable data on relative partial ionization cross
sections and momentum spectra of fragment ions for N2O and
dissociation dynamics of ions of CO2 molecule [281- 283]. The
experimental facility, for investigations on fragmentation dynamics of
molecular ions relevant to edge-plasma ion-surface interaction, is the first of
its kind in the university system of India and was funded by the National
Fusion Program of the country
5.4 Laser Induced Breakdown Spectroscopy (LIBS)
In
LIBS experiments a Laser beam, focused on a sample (solid, liquid or gas),
generates a spark by converting a tiny amount of material into plasma whose
emission is recorded by a spectrometer. The spectrum belongs to atoms and ions
of the elements that are present in the sample. Although the first reports of
the analytical use of laser-induced plasma were published in early 1960s, the
widespread use of this technique was made possible with advances in gated
optical detectors during the 1980s and early 1990s. During the past two decades
LIBS has found applications in almost every branch of sciences including
archeology, nuclear waste management and analysis of geological samples. LIBS
has emerged as a very powerful and
versatile analytical tool due to its capability of rapid, in-situ measurement,
simultaneous detection of all elements present in the periodic table and real
time analysis of materials in the laboratory or in the field. The ability to
detect molecular and elemental signatures with a single laser pulse offers
unprecedented advantages for emerging medical, biological, environmental and
security applications. A typical experimental arrangement for recording LIBS
signals from a solid sample is shown in Fig.12 where the sample is put on a
rotating platform to avoid excessive pit formation at one point on the sample
due to the pulsed laser beam ablation.
Experiments have been carried out in the spectroscopy
laboratory of Prof. A K Rai at the Allahabad University on solid as
well as liquid samples. LIBS investigations on a cross –section of gall-blader
stone has revealed the mechanism of stone formation in human organs [284]. It
has been possible to identify nitro-compounds on the basis of their relative
atomic contents by LIBS [285] and presence of chromium in water has been
detected with very high sensitivity [286]. LIBS has also been used as a
diagnostic tool for rare earth doped glasses [287] and for contaminant
concentration in environmental samples [288].
6.
Concluding Remarks
It can be seen from a
perusal of the above sections of this article that the spectroscopic
instrumentation at BHU has had a long and tedious journey starting with
homebuilt and low resolution commercial spectrometers to very high resolution
grating spectrographs. The spectroscopic sources consisting of flames, electric
arcs and discharge tubes gave way to microwave discharges and eventually to
tunable laser sources. The detection of spectra progressed from human eye to
photographic films, photomultiplier tubes and eventually to CCD detectors. The
samples investigated by spectroscopic techniques in this laboratory started
with simple salts to be burnt in a flame or an electric arc to liquids and
solutions which could be examined by absorption spectroscopy or vaporized to
emit spectra in discharge tubes. With the introduction of lasers in the
laboratory, it has now become possible to investigate solid, liquid and vapour
samples with equal ease. The theoretical research that started with force field
and potential curve calculations on mechanical calculating machines has now
reached the sophistication of potential surfaces and quantum mechanical methods
and calculations carried out on very fast digital computers. All this
development has required the persistent efforts from almost four generations of
students, research scholars and teachers. I have had the privilege of
association with this famous laboratory for more than 50 years and during this
period more than a thousand persons have acquired postgraduate and doctoral
skills. It is not possible to remember them all but without any prejudice I
would like to name a few, in the following section, who have maintained contact
even after their formal departure from the Spectroscopy Laboratory of BHU and
who have had a very distinguished career.
Dr S K Tiwari as
well as Dr R B Singh, who had ushered the laboratory into era of high
resolution spectroscopy by installing the large grating spectrograph, excelled
in their professions. Dr Tiwari translated and wrote many books on physics in
the Hindi publication board at BHU and continues to serve the cause of
education at the ripe age of 80. Dr Singh joined the Central Forensic Service
in Govt. of India and retired as Director of State Forensic Laboratory of Uttar
Pradesh. Dr R N Singh and Dr D P Jual had joined IRDE of the Ministry of
Defense at Dehradun and distinguished themselves in the areas remote sensing
and laser applications respectively. Dr R J Singh and Dr P K Verma joined AMU
at Aligarh and became specialized professors in the fields of magnetic
resonance spectroscopy and vibrational spectroscopy respectively. Dr G Joshi
and much later Dr V K Rai have distinguished themselves in X-ray spectroscopy
and in laser spectroscopy respectively at IMS Dhanbad. Dr A N Singh started
research in quantum chemistry of molecules and helped Dr S N Singh also in the
same area to become professors at the Magadh University in Bodh Gaya. Dr K P R
Nair has made immense contributions in microwave spectroscopy of molecules
while working in Germany and at the Cochin University of Science and
technology. Dr G D Baruah established spectroscopy laboratory in Dibrugarh
University and Dr S N Rai has worked at NEHU in Meghalaya. Dr S Rai, who joined
Dibrugarh University much later and excelled in photoacoustic spectroscopy
while working with Professor Baruah, is now professor of physics at Mizoram
University. Dr Sri Ram Singh, who started the zone refined technique for making
single crystals of organic molecules, has served the Central Bureau of
Investigation with great distinction in forensic science. Dr Jagdish Singh, at
the Directorate of Satellite Meteorology in Jammu, faced a terrorist attack in
his station with utmost bravery and has retired after rendering valuable service
to the organization. Dr M M Rai who had worked on the vibrational spectroscopy
of benzene derivatives rose to the position of research manager in Indian Oil
Corporation. Dr J S Yadav, who was in the first group to start quantum chemical
calculations on large molecules, has done excellent work on biomolecules and
has settled in USA. From the group that
started theoretical research in atomic and molecular collisions Professor D N Tripathi retired after a long association with this laboratory, both Dr B N Roy
and Dr S N Tiwary became professors at BRA University in Muzaffarpur, Dr Rajesh
Srivastava became professor at IIT Roorkee and Dr B N Padhy continues to work
in association with Institute of Physics at Bhubaneshwar after retirement from
the B.J.B. College. From the group that was involved in developing laboratory
facilities in collision spectroscopy, Dr S K Goel is Scientist E at DST, New
Delhi and Dr M J Singh is a Scientist F at IPR, Ahmedabad. Dr B R Yadav, an
expert in high resolution spectroscopy, has served as the Deputy Director of
the prestigious ONGC Titan Program. Dr J P Singh who was instrumental in
building lasers and developing laser based spectroscopy techniques has
distinguished himself in many applications of Coherent Antistokes Raman Spectroscopy
(CARS) and Laser Induced Breakdown Spectroscopy (LIBS). He has continued to
serve the cause of modernizing his alma mater and has helped me start working
on a variety of LIBS investigations. Dr V N Rai, who was involved in developing
photoacoustic spectroscopy at BHU, works on many applications of laser plasma
and laser-matter interaction at RRCAT in Indore. Dr Shanker Ram, who carried
out IR and Raman spectroscopy of molecules, has done path breaking research in
materials and is an eminent professor in Material Science at IIT, Kharagpur. Dr
A K Rai who carried out the first experiments on laser optogalvanic
spectroscopy at BHU is now professor of physics at Allahabad University and has
used photoacoustic spectroscopy as well as LIBS for many investigations of
great importance in Environmental Science, Agriculture and Medicine. Dr R S
Ram, who worked on high resolution electronic spectroscopy of diatomic
molecules, has emerged as an international expert on heavy diatomics while
working at the Arizona State University in USA. Dr V B Singh and his research
scholars, at UP College in Varanasi, have worked on experimental and
theoretical problems on molecules of great importance in Earth’s atmosphere and
in astrophysics. Dr S K Srivastava, from the first group that initiated
experimental and theoretical spectroscopic studies on biomolecules, has been a
leading academic functionary including director at the Institute of Engineering
and Technology, Lucknow. Dr R A Singh is a professor and former head of physics
department at H. S. Gaur University, Sagar and Dr O P Singh has continued his
researches in quantum chemistry of molecules at the Balarampur PG College. Dr B
N Singh is in laser instrumentation at RRCAT and Dr Santosh C. is professor at
the center for atomic and molecular physics in Manipal University. Dr Narayanan
K is presently chairman of department of Physics & Astronomy at College of
Charleston, SC in USA and has been working on photoacoustic spectroscopy and
nanomaterials. Dr K K Mahato is now a professor in Biophysics Unit of Manipal
University and has been carrying out quality research on photoacoustics of
biological materials. Dr R C Sharma is presently at Laser science and
Technology Centre of DRDO under the Ministry of Defense and Dr R L Prasad is in
the Physics department of Eritrea Institute of Technology in NE Africa. Dr
Akshay Kumar, who carried out a variety of investigations on RE ion doped
glasses, is presently in the Physics department of Tuskegee University, AL in
USA.
It is very heartening to talk to the present group of
research scholars in the Laser and Spectroscopy Laboratory. Most of them appear
very keen to put their research findings to some kind of practical
applications. This is a big change in the attitude from that of early 1960s when
the workers did not bother about applications and most of the attention was
focused on basic research as well as improving the experimental facilities.
This change of attitude is good for technological developments in addition to
the scientific knowledge. At the time of the 50th anniversary of
Spectroscopy at BHU the number of teachers was 12 and it is sad to find that
this number has dropped to almost half at the 75th anniversary. The
university administration, with much better communication facilities of the
internet, is expected to keep the spirit of research in full swing by timely reinforcing
faculty as well as technical manpower. I would take this opportunity to make a
suggestion for revising the PG Diploma course in spectroscopy. The techniques
of Multi Spectral Imaging and Hyper Spectral Imaging need to be included in
this course in addition to specialized software for interpretation of these
images. It would help students to develop expertise in analyzing the imaging
data from Earth Resources Satellites and also those from the modern day
microscopes being used in biology and medicine.
Acknowledgements
During
my brief visit to BHU in December 2013 some of our old and new spectroscopy
students suggested to me that it would be good if I could write a brief history
of our famous spectroscopy laboratory. Prof. S B Rai provided me with a lot of
scientific literature both current and of the past. Prof. P C Mishra was very
prompt in sending me his recent papers when I found myself inadequate in that
area. Dr Kavita Mishra sent the photographs of the grating spectrograph and
meticulously read through the first draft of the article. Ms Ranjana Singh
provided the much needed photo of late Prof. B P Asthana. Professor R. Shanker
also provided photographs and some information about our former students. Ms
Shashi Kiran Singh, one of our former
students, found time from her busy schedule to read and make significant improvements
in the manuscript. I have borrowed a large amount of material from a
publication on the occasion of 50th anniversary of the laboratory in
which all the teachers had contributed articles. I have tried to be as
objective as possible in presenting the research activities from my personal
knowledge but my inadequacy with theoretical aspects may be reflected in
somewhat greater details of describing the experimental activities. I have been
very privileged to have excellent support from my family, Dr Punam and Dr
Vineeta have taken care of my health, Rudra, Sudheer and Sangeeta have provided
excellent support on the computer in writing this article and Tanya has helped
with its presentation. I have spent so much time in BHU that I have come to
love it as much as I love Gangauli, the village, where I was born. My Ph.D
supervisor Prof. K N Upadhya was so kind and affectionate to me that no words
can express my sense of gratitude to him and this article is dedicated to him
as a mark of my profound respect.
References
1. N.L.Singh, Spectroscopy
Laboratory-50th Anniversary Publication, Department of Physics, BHU,
December 11-15 (1989) page 1
2. P. Venkateswarlu, Phys. Rev. 81 (1951) 821
3. R.K. Asundi and M.R. Padhye,
Nature 156 (1945) 368
4. B.D.Joshi, Spectroscopic Studies
of p-dichlorobenzene, benzonitrile and chlorophenol, Ph.D. Thesis, BHU (1955)
5. I.S. Singh, Spectroscopic Studies
of isomeric di-methylbenzenes, Ph.D. Thesis, BHU (1956)
6. P.R.K. Rao, Spectroscopic Studies
of isomeric cresols and phenetole, Ph.D. Thesis, BHU (1958)
7. K. N. Upadhya, Near UV Spectra of fluorobenzene,
ethylbenzene, o-chlorobenzene, p-chlorophenol and benzotrifluoride, Ph.D.
Thesis, BHU (1959)
8. S.N. Thakur, D.K. Rai and N.L.
Singh, J. Chem. Phys., 48 (1968)
3389
9. N.L.Singh, Canad. J. Phys. 37 (1959) 136
10. B.S. Mohanty, K.N. Upadhya and
D.K. Rai, Ind. Acad. Sci. A LXVIII (1968) 165
11. S.B. Rai, D.K. Rai and K.N.
Upadhya, J. Phys. B 5 (1972) 1038
12. R.B. Singh and S.K. Tiwari, BHU
J. Scientific Research (1963)
13. R.B. Singh, Ph. D. Thesis,
Banaras Hindu University (1966)
14. R.Tripathi, K.N. Upadhya, R.B.
Singh and S.B. Rai, J. Phys. B15 (1982)
4393
15. R. Shanker, O.N. Singh and I.S.
Singh, Canad. J. Phys. 47 (1969)
1601
16. V.S. Kushwaha, B.P. Asthana and
C.M. Pathak, J. Mol. Spectrosc. 41 (1972)
577
17. K.P.R. Nair and D.K. Rai, Canad.
J. Phys. 45 (1967) 2810
18. K.P.R. Nair and K.N. Upadhya,
Nature 211 (1966) 1170
19. K.P.R. Nair and K.N. Upadhya, Canad.
J. Phys. 44 (1966) 2241
20. R.K. Pandey, K.N. Upadhya and
B.S. Mohanty, Ind. J. Phys. 42 (1968)
154
21. G.P. Mishra, R. Tripathi, S.B.
Rai, K.N. Upadhya and D.K. Rai, J. Mol. Spectrosc. 85 (1981) 243
22. G.P. Mishra, S.B. Rai and K.N.
Upadhya, Canad. J. Phys. 59 (1981)
289
23. D.K. Rai, Ph.D. Thesis, Banaras
Hindu university (1966)
24. R.S. Becker, I.S. Singh and E.A.
Jackson, J. Chem. Phys. 38 (1963)
2144
25. V.B. Singh, R.N. Singh and I.S.
Singh, Spectrochim. Acta 22 (1966)
927
26. G. Joshi and N.L. Singh,
Spectrochim. Acta 22 (1966) 1501
27. V.B. Singh and I.S. Singh, Curr.
Sci. 36 (1967) 365
28. M.P.Srivastava, Electronic
spectra of isomeric fluorobenzaldehydes, Ph.D. Thesis, BHU (1967)
29. A.N. Singh, Ph.D. Thesis, Banaras
Hindu University (1967)
30. V.B. Singh, Ph.D. Thesis, Banaras
Hindu University (1967)
31. B.B. Lal, Ph.D. Thesis, Banaras
Hindu University (1973)
32. S.R. Singh, Ph.D. Thesis, Banaras
Hindu University (1973)
33. Shyam Pati, I.S. Singh and J.
Shamir, Ind. J. Phys. 51B (1977) 50
34. R.B. Singh and D.K. Rai, Canad.
J. Phys. 43 (1965) 167
35. S.N. Thakur and D.K. Rai, J. Mol.
Spectrosc. 19 (1966) 341
36. S.N. Thakur and S.N. Rai, J. Mol.
Struct. 5 (1970) 320
37. S.N. Thakur, J. Mol. Struct. 7 (1971) 315
38. C.M. Pathak and W.H. Fletcher, J.
Mol. Spectrosc. 31 (1969) 32
39. R.A. Yadav, I.S. Singh and O.
Sala. J. Raman Spectrosc. 14 (1983)
353
40. N.G. Dongre, Ph.D. Thesis,
Banaras Hindu University (1985)
41. A.R. Shukla, C.M. Pathak, N.G.
Dongre, B.P. Asthana and J. Shamir, J. Raman Spectrosc. 17 (1986) 299
42. B.P. Asthana and C.M. Pathak,
Spectrochim. Acta. 41A (1985) 595
43. B.P. Asthana and C.M. Pathak,
Spectrochim. Acta. 41A (1985) 1235
44. S.N. Thakur, L. Goodman, A.G.
Ozkabek, J. Chem Phys. 84 (1986)
6642
45. K. Manihar Singh, Ph.D. Thesis,
Banaras Hindu University (1988)
46. R.A. Yadav, P.N.S. Yadav and J.S.
Yadav, Spectrochim. Acta 44A (1988)
1201
47. K.S. Pandey, B.P. Asthana and
P.C. Mishra, Spectrochim. Acta 49A (1993)
53
48. K. Singh, R.A. Yadav and J.S.
Yadav, Spectrochim. Acta 47A (1991)
819
49. R.A. Yadav, I.S. Singh and O.N.
Singh, J. Raman Spectrosc. 23 (1992)
141
50. R.A. Yadav, Spectrochim. Acta 49A
(1993) 891
51. R. Shanker, R.A. Yadav and I.S.
Singh, Spectrochim Acta 50A (1994)
1251
52. D.N. Singh, R. Shanker, R.A.
Yadav and I.S. Singh, J. Raman Spectrosc. 27 (1996) 177
53. D.N. Singh, J.S. Singh and R.A.
Yadav, J. Raman Spectrosc. 28 (1997)
355
54. V.K.Rastogi, C.B.Arora,
R.A.Yadav, C.Singh and M.A.Palafox, J.Raman Spectro 31 (2000)595
55. J.N. Rai, Spectroscopic studies
of some polyatomic molecules,Ph.D. Thesis, BHU (1967)
56. P.C. Upadhya, Spectral studies of
substituted benzenes and substituted diazenes, Ph. D. Thesis, BHU (1974)
57. S. N. Thakur and N.L. Singh, Ind.
J. Pure & Appl. Phys. 7 (1969)
765
58. S. N. Thakur and K.N. Upadhya, J.
Mol. Struct. 4 (1969) 459
59. P.H. Hepburn, J.M. Hollas and
S.N. Thakur, Mol. Phys. 29 (1975)
637
60. R.K. Asundi, B. Bhattacharya and
N.A. Narasimham, Curr. Sci. 21 (1952)
273
61. S.N. Garg, Current Science 23 (1954) 150
62. R.S. Singh, Absorption spectrum of
benzoquinone, Ph.D. Thesis, BHU (1955)
63. D.P. Juyal, Electronic spectra of
isomeric chlorobenzaldehydes, Ph.D.Thesis, BHU (1966)
64. S.N. Thakur, Ind. J. Phys. 51B (1977) 184
65. M.K. Haque and S.N. Thakur, Chem.
Phys. Lett. 66 (1979) 561
66. M.K. Haque and S.N. Thakur, J.
Mol. Struct. 57 (1979) 163
67. M.G. Jaiswal, Sensitised
triplet-singlet emission of quinines in the vapour phase, Ph.D. Thesis, BHU (1965)
68. V.N. Pandey and S.N. Thakur,
Proc. Ind. Acad. Sci. (Chem.Sci.)92 (1983)
127
69. S.Ram,V.N. Pandey & S.N. Thakur, Pramana, 20 (1983) 163
70. S.N. Thakur and K.K. Innes, J.
Mol. Spectrosc. 52 (1974) 130
71. J.M. Hollas and S.N. Thakur, Molec. Phys. 22 (1971) 203
72. R.D. Singh and R.S. Singh, Ind.
J. Pure & Appl. Phys. 3 (1965)
418
73. R.D. Singh and R.S. Singh, Ind.
J. Pure & Appl. Phys. 4 (1966)
263
74. R.A. Singh and S.N. Thakur, Mol.
Phys. 36 (1978) 1053
75. R.A. Singh and S.N. Thakur, J.
Cryst. Mol. Struct. 11 (1981) 197
76. S. Nath Singh, Spectral studies
on naphthaquinones and anthraquinones, Ph. D. Thesis, BHU (1968)
77. G.D. Baruah, Ph. D. Thesis,
Banaras Hindu University (1972)
78. P.C. Mishra and D.K. Rai, Int. J.
Quant. Chem. VI (1972) 47
79. P.C. Mishra and D.K. Rai, Ind. J.
Pure & Appl. Phys. 11 (1973) 32
80. P.C. Mishra, S.N. Thakur and D.K.
Rai, J. Cryst. Mol. Struct. 1 (1971)
99
81. P.C. Mishra and D.K. Rai, Mol.
Phys. 23 (1972) 631
82. P.C. Mishra and D.K. Rai, Mol.
Phys. 23 (1972) 815
83. J.S. Yadav, P.C. Mishra and D.K.
Rai, Chem. Phys. Lett. 19 (1973) 445
84. J.S. Yadav, G. Barnickel, H.
Labischreinski and H. Bradaczek, J. Theor. Biol. 88 (1981) 441; ibid 95 (1982)
151, 167
85. J.S. Yadav and P. Luger, Int J.
Quant. Chem. 23 (1982) 1433, 1441
86. P.N.S. Yadav, J.S. Yadav and D.K.
Rai, J. Mol. Struct.(Theochem) 194 (1989)
19
87. J.P. Singh and S. N. Thakur,
Research & Industry 23 (1978)
227
88. J.P. Singh and S.N. Thakur,
Applied Optics 19 (1980) 1697
89. J.P. Singh, Studies on High gain
Lasers, Ph. D. Thesis, BHU (1980)
90. C. Lal and S. N. Thakur, Applied
Optics 21 (1982) 2317
91. C. Lal and S. N. Thakur, Applied
Optics 22 (1983) 770
92. B.N. Singh, Fabrication,
Chraracterization and Spectral Studies of N2 laser pumped Dye
Lasers, Ph. D. Thesis, BHU (1987)
93. S.B. Rai, and D.K. Rai, Chem.
Phys. Lett. 80 (1981) 605
94. G.P. Mishra, S.B. Rai and K.N.
Upadhya, Canad. J. Phys. 57 (1979)
824
95. B. Hemmerling, S.B. Rai and W.
Demtroder, Z. fur Physik A320 (1985)
135
96. S.B. Rai, B. Hemmerling and W.
Demtroder, Chem. Phys. 97 (1985) 127
97. S.R. Govil, D.C. Agrawal, K.P. Rai and S.N. Thakur, Proc. Ind.
Nat. Acad. B49 (1983). 71
98. S.N. Thakur, J.G. Philis and L. Goodman, Chem. Phys. Lett. 95 (1983) 290
99. S.N. Thakur and L. Goodman, J. Chem. Phys. 78 (1983) 4356
100. T.K. Kundu, S.N. Thakur
and L. Goodman, J. Chem. Phys. 97 (1992)
5410
101. I.B. Singh and S.B. Rai J. Sci. Res. 42 (1992) 135
102. I.B. Singh, S.B.Rai and D.K. Rai J. Mol. Spectr. 163 (1994) 364
103 I.B. Singh, S.B. Rai and D.K. Rai Pramana, 45 (1995) 271
104. K.K. Mahato, S.B. Rai and D.K. Rai Ind. J. Phys. 69B (1995) 27
105. V.N.
Rai, J.P. Singh and S.N. Thakur, Research & Industry 26 (1981) 165
106. A.G.
Bell, ‘Upon the Production of Sound by Radiant Energy’, Phil. Mag. J. Sci.
XI (1881) 510
107. L.B.
Tiwari, Photoacoustic spectroscopy of some materials in condensed phase, Ph. D.
Thesis, BHU (1984)
108. V.N.
Rai, S.N. Thakur and D.K. Rai, Applied Spectroscopy 40 (1986) 1211
109. S.
Rai, S.B. Rai and S.N. Thakur, Spectrochim. Acta A44 (1988) 1473
110. V.N.
Rai, S.N. Thakur and D.K. Rai, Journal of Phys. E20 (1987) 1472
111. V.N.
Rai, B.N. Singh and S.N. Thakur, Applied Optics 28 (1989) 651
112. S. Rai, A.K. Rai and
S.N. Thakur, Ind. J. Pure & Appl. Phys. 26 (1988) 649
113. K.
Narayanan and S.N. Thakur, Appl. Opt. 29 (1990)
2478
114. K.
Narayanan and S.N. Thakur, Appl. Optics 30 (1991) 1175
115. R.L.Prasad,
R.Prasad,G.C.Bhar,S.N. Thakur, Spectrochim Acta A58 (2002) 3093
116. C. Lal and S.N. Thakur,
Research and Industry 31 (1988) 124
117. K. Narayanan and S.N.
Thakur, Appl. Optics 31 (1992) 4987
118. S.N. Thakur,
D.Guo,T.K.Kundu and L.Goodman, Chem.PhysLett.199 (1992) 335
119. C. Hornberger, M. Konig, S.B.Rai and
W.Demtroder, Chem. Phys. 190 (1995)
171
120. Vipin Prasad J., S.B. Rai and S.N. Thakur,
Chem. Phys. Lett. 158 (1989) 626
121. R. Gross Klass, S.B. Rai, R. Stuber and W.
Demtroder, Chem. Phys. Let. 229 (1994)
609
122. I.B. Singh, S.B. Rai and S.N. Thakur, Ind. J.
Phys. 69B (1995) 315
123. Vineet Kumar Rai, D.K. Rai and S.B. Rai,
Spectrochim Acta A59 (2003) 1299
124. A. Rai, D.K. Rai and S.B. Rai, Spectrochim
Acta A60 (2004) 53
125. Garima Tripathi, Vineet kumar Rai and S.B. Rai,
Ind. J. Phys. 78 (2004) 1377
126. Y. Dwivedi, S.N. Thakur and S.B. Rai,
Spectrochim. Acta A71 (2009) 1952
127. R.C.
Sharma and S.N. Thakur, PRAMANA 56 (2001)
87
128. S.S.
Joshi, Current Science 8 (1939) 548
129. S.S.
Joshi and V. Narasimhan, Current Science 9 (1940) 535
130. S.S.
Joshi and G.S. Deshmukh, Nature 155 (1945)
483
131. N.A.
Narasimham, Ind. J. Phys. 26 (1952)
512
132. P.M.
Penning, Physica 8 (1928) 37
133. A.K.
Rai, S.B. Rai, S.N. Thakur and D.K. Rai, Chem. Phys. Lett. 138 (1987) 215
134. V.
Kumar, A.K. Rai and D. K. Rai, PRAMANA
29 (1987) 163
135. V.
Kumar, A.K. Rai and D.K. Rai, PRAMANA 31
(1988) L421
136. V.
Kumar, A.K. Rai, S.N. Thakur and D.K. Rai, Chem. Phys. Lett. 142 (1987) 217
137. G.
Ullas, S.B. Rai and D.K. Rai, J. Phys. B25 (1992) 4497
138. G.
Ullas, V. Kumar and S.B. Rai, PRAMANA 39
(1992) 359
139. K.
Narayanan, G. Ullas and S.B. Rai, Chem. Phys. Lett. 155 (1989) 55
140. S.N. Thakur and K.
Narayanan, Optics Commun. 94 (1992)
59
141. S.B. Rai and D.K. Rai Ind. Nat. Sci. Acad. 62A (1996) 475
142. B.P. Asthana and W. Kiefer, Appl. Spectrosc. 36 (1982) 250
143. B.P. Asthana, H. Takahashi and W. Kiefer, Chem. Phys. Letters 94 (1983)
41
144. B.P.
Asthana and W. Kiefer, Appl. Spectrosc. 37 (1983) 334
145. H.
Takahashi, B.P. Asthana, W. Häfner and W. Kiefer, J. Raman Spectrosc 14 (1983) 102
146. A.R.
Shukla, C.M. Pathak. N.G. Dongre, B.P. Asthana and J. Shamir, J. Raman
Spectrosc. 17 (1986) 299
147. B.P.
Asthana and W. Kiefer, Vibrational Spectra & Structure (J.R. Durig Editor)
20 (1993) 67
148. V.
Deckert, B.P. Asthana, W. Kiefer, H.-G.
Purucker and A. Laubereau, J. Raman Spectrosc. 31 (2000) 805
149. S.
Schlucker, R.K. Singh, B.P. Asthana, J. Popp and W. Kiefer, J. Phys. Chem. A105
(2001) 9983
150. S.
Schlucker, M. Heid, R.K. Singh, B.P. Asthana, J. Popp and W. Kiefer, Z. Phys.
Chem. 216 (2002) 267
151. R.K.
Singh, S. Schlucker, B.P. Asthana and W. Kiefer, J. Raman Spectrosc. 33 (2002) 720
152. R.K.
Singh, S. Schlucker, B.P. Asthana and W. Kiefer, Applied Spectrosc. 57 (2003) 1288
153. S.K.
Srivastava, A.K. Ojha, J. Koster, M.K. Shukla, J. Leszczynski, B.P. Asthana and W. Kiefer, J. Mol. Structure 661-662 (2003) 11
154. A.K.
Ojha, S.K. Srivastava, N. Peica, I. Pavel, W. Kiefer and B.P. Asthana, J. Mol.
Structure 735-736 (2005) 349
155. S.K.
Srivastava, A.K. Ojha, B.P. Asthana and W. Kiefer,, Spectrochim Acta A61 (2005) 2832
156. A.G.
Ozkabak, S.N. Thakur & L. Goodman, Int. J. Quant. Chem. 39 (1991) 411
157. R. Shanker, R. A. Yadav, I. S. Singh and O. N. Singh PRAMANA 24 (1985) 749
158. R. A. Yadav Proc. Ind. Acad. Sci. (Chem. Sci.) 102 (1990) 629
159. D.N. Tripathi and D.K. Rai, J. Phys. B3 (1970) 365
160. D.N. Tripathi and D.K. Rai, J. Quant.
Spectrosc. & Rad. Transfer 10 (1970)
1329
161. B.N.
Roy, D.N. Tripathi and D.K. Rai, Phys. Rev. A5 (1972) 1252
162. B.N.
Roy and D.K. Rai, J. Phys. B12 (1979)
2015
163. S.N.
Tiwary and D.K. Rai, J. Phys. B9 (1976)
63
164. R.
Srivastava and D.K. Rai, J. Phys. B10 (1977)
269
165. R.
Srivastava and D.K. Rai, Phys. Rev. A15 (1977)
1906
166. C.S.
Singh and S.N. Rai, Phys. Rev. A33 (1986)
4390
167. B.N.
Padhy and D.K. Rai, Phys. Rev. A37 (1988)
3738
168. B.N.
Padhy, Ph.D. Thesis, Banaras Hindu University (1988)
169. W.
Jitschin, R. Hippler, R. Shanker, H. Kleinpoppen, R. Schuch and H.O. Lutz, J.
Phys. B16 (1983) 1417
170. K.N.
Pandey, R. Shanker, D.N. Tripathi and D.K. Rai, Ind. J. Tech. 25 (1987) 393
171. K.N.
Pandey, R. Shanker and D.N. Tripathi, Ind. J. Phys. 63B (1989) 47
172. S.K.
Srivastava and P.C. Mishra, Int. J. Quant. Chem. 16 (1979) 1051
173. S.K.
Srivastava and P.C. Mishra, Int. J. Quant. Chem. 18 (1980) 827
174. S.K.
Srivastava and P.C. Mishra, J. Mol. Struct. 65 (1980) 199
175. S.K.
Srivastava and P.C. Mishra, J. Theoret. Biol. 93 (1981) 655
176. P.C.
Mishra and R.D. Tiwari. PRAMANA 26 (1986)
505
177. P.C.
Mishra, J. Mol. Struct. 144 (1986)
309
178. P.C.
Mishra, J. Mol. Struct. 195 (1989)
201
179. Santhosh,
C. and P.C. Mishra, J. Mol. Struct. 196 (1989)
327
180. P.C.
Mishra and K.S. Pandey, Proc. Ind. Acad. Sci. (Chem. Sci.) 101 (1989) 65
181. Santhosh,
C. and P.C. Mishra, J. Mol. Struct. 220 (1990)
25
182. Santhosh,
C. and P.C. Mishra, J. Photochem. Photobiol. 51 (1990) 245
183. C. Santhosh
and P.C. Mishra, Spectrochim. Acta, 47A (1991) 1685
184. M.K. Shukla and P.C. Mishra, Spectrochim. Acta, 51A (1993) 831
185. C.G. Mohan, A. Kumar, and P.C. Mishra, Int. J. Quantum
Chem. 60 (1996) 699
186. C. Santhosh, M.K. Shukla and P.C. Mishra, P.C., J. Mol.
Model. 4 (1998) 250
187. P.C. Mishra and A. Kumar, Int. J. Quantum Chem. 71 (1999) 191
188. S.K. Mishra, M.K. Shukla
and P.C. Mishra, Spectrochim. Acta A56 (2000)
1355
189. M.K. Shukla, S.K.
Mishra, A. Kumar and P.C. Mishra, J. Comput. Chem. 21 (2000) 826
190. N.R. Jena and P.C.
Mishra, J. Phys. Chem. B 109 (2005)
14205
191. P.K. Shukla and P.C. Mishra, J. Phys. Chem. B112 (2008) 4779
192. N.R. Jena, P.C. Mishra and
S. Suhai, J. Phys. Chem. B113 (2009) 5633
193. Neha Agnihotri and P.C. Mishra, J.
Phys. Chem. B114 (2010) 7391
194. Neha Agnihotri and P.C. Mishra, J.
Phys. Chem. A115 (2011) 14221
195. K.K. Mahato and S.B. Rai, Spectrochim. Acta
A56 (2000) 2333
196. Akshay Kumar, D.K. Rai and S.B. Rai, Solid
State Commun. 117 (2001) 387
197. Akshay Kumar, S.B. Rai and D.K. Rai,
Spectrochim Acta A58 (2002) 1379
198. S.B. Rai, Spectrochim Acta, A58 (2002) 1559
199. Akshay Kumar, D.K. Rai and S.B. Rai, Spectrochim
Acta A58 (2002) 3067
200. K.K. Mahato, S.B. Rai and A. Rai, Spectrochim.
Acta, A60 (2004) 979
201. K.K. Mahato, A. Rai and S.B. Rai, Spectrochim.
Acta A61 (2005) 431
202. Akshay Kumar, D.K. Rai
and S.B.Rai, Materials Res. Bull. 38 (2003)
333
203. Y. Dwivedi and S.B. Rai,
Opt. Mater. 31 (2008) 87
204. Priyanka Srivastava,
D.K. Rai and S.B. Rai, Spectrochim. Acta A59 (2003) 3303
205. Priyanka
Srivastava, S.B. Rai and D.K. Rai, Spectrochim Acta A60 (2004) 637
206. Priyanka
Srivastava, S.B. Rai and D.K. Rai, J. Alloys & Compds. 368 (2004) 1
207. A.
Bahadur, Y. dwivedi and S.B. Rai, Spectrochim. Acta A110 (2013) 400
208. Akshay
Kumar, D.K. Rai and S.B. Rai, A58 (2002)
2115
209. Akshay
Kumar, D.K. Rai and S.B. Rai, A59 (2003)
917
210. S.B.
Rai, A.K. Rai and S.K. Singh, Spectrochm. Acta A59 (2003) 3221
211. V.K.
Rai, D.K. Rai and S.B. Rai, J. Mat. Sci. Lett. 39 (2004) 4971
212. V.K.
Rai, D.K. Rai and S.B. Rai, Spectrochim. Acta A62 (2005) 302
213. V.K.
Rai and S.B. Rai, Solid State Commun. 132 (2004)
647
214. A.K.
Singh, V.B. Singh and S.B. Rai, J. Alloys & Compd 404 (2005) 97
215. A.K.
Singh, D.K. Rai and S.B.Rai, Solid State Commun. 136 (2005) 346
216. A.K.
Singh, D.K. Rai and S.B.Rai, J. Appl. Phys. 82 (2006) 289
217. V.K.
Rai, D.K. Rai and S.B. Rai, Opt. Commun. 257 (2006) 112
218. V.K.
Rai, K.Kumar and S.B. Rai, Opt. Materials. 41 (2006) 151
219. Garima
Tripathi, V.K. Rai and S.B. Rai, Spectrochim. Acta. A62 (2005) 1120
220. Y.
Dwivedi, S.N. Thakur and S.B. Rai, Appl. Phys. B89 (2007) 45
221. N.K.
Giri, A.K. Singh and S.B. Rai, J. Appl. Phys. 101 (2007) 33102
222. Garima
Tripathi, V.K. Rai and S.B. Rai, Optical Materials 30 (2007) 201
223. A.K.
Singh, Garima Tripathi, A. Rai and S.B. Rai, J. Appl. Phys. 101 (2007) 103105
224. Kavita
Mishra, Y. Dwivedi and S.B. Rai, Appl. Phys. B106 (2012)
101
225. Kavita
Mishra, N.K. Giri and S.B. Rai, Appl. Phys. B103 (2011) 863
226. Y.
Dwivedi, Kavita Mishra and S.B. Rai, J. Alloys & Compds. 572 (2013) 90
227. P.C.
Mishra and Amarjeet Yadav, Chem Phys. 402 (2012)
56
228. P.C.
Mishra and A. Yadav, J.Chem. Phys. A117 (2013)
8958 ; Correction 10566
229. R.A.
Yadav, V. Mukherjee, M. Kumar and Rashmi Singh, Spectrochim. Acta.
A 66 (2007) 964
230. R.L.
Prasad, A. Kushwaha, Suchita, M. Kumar and R.A. Yadav, Spectrochim. Acta. A69 (2008) 304
231. R.A.
Yadav, M. Kumar, R. Singh, P. Singh, S. Jaiswal, G. Srivastav and R.L. Singh,
Spectrochim. Acta A71 (2008) 1565)
232. S.
Jaiswal, A. Kushwaha, R. Prasad, R.L. Prasad and R.A. Yadav, Spectrochim. Acta.
A74 (2009) 16
233. R.L.
Prasad, A. Kushwaha, R. Prasad, S. Jaiswal and R.A. Yadav, J. Theo. Comp. Chem.
8 (2009) 1485
234. R.
Singh, S. Jaiswal. M. Kumar, P.Singh, G. Srivastav and R.A. Yadav, Spectrochim.
Acta. A75 (2010) 267
235. S.
Jaiswal, Deepshikha Singh, R.L. Prasad and R.A. Yadav, Spectrochim. Acta. A76 (2010) 297
236. I.D.
Singh and R.A. Yadav, PRAMANA 62 (2004)
1255
237. R.A.
Yadav, T.K.Yadav,M.K.Maurya, D.P.Yadav and N.P.Singh, J.Phys. 83 (2009) 1421
238. M.K.
Maurya, T.K. Yadav and R.A. Yadav, PRAMANA 72 (2009) 709
239. M.K.
Maurya, T.K. Yadav and R.A. Yadav, Opt. Laser Tech. 42 (2010) 465
240. M.K.
Maurya, T.K. Yadav, Ruchi Singh, R.A. Yadav and D.P. Singh, Opt. Commun. 283 (2010) 2416
241. M.K.
Maurya, T.K. Yadav and R.A. Yadav, Opt. Laser Tech. 42 (2010) 775
242. S.K.
Singh, K. Kumar and S.B. Rai, Sens. Actu. A149 (2009) 149
243. S.K.
Singh, K. Kumar and S.B. Rai, Appl.Phys. B94 (2009) 165
244. K.Kumar,
R.N. Rai and S.B. Rai, Appl. Phys. B96 (2009)
96
245. S.K.
Singh, K. Kumar and S.B. Rai, J. Appl. Phys. 106 (2009) 093520
246. S.K.
Singh, K.Kumar, M.K. Srivastava, D.K. Rai, S.B. Rai, Opti. Lett. 35 (2010) 175
247. A.
Bahadur, Y. Dwivedi and S.B. Rai, Spectrochim. Acta A77 (2010) 101
248. Y.
Dwivedi, A. Bahadur and S.B. Rai, J. Non-Cryst. Solids 356 (2010) 1650
249. Y.
Dwivedi, A. Bahadur and S.B. Rai, J. Appl. Phys. 110 (2011) 043103
250. A.
Bahadur, Y. dwivedi and S.B. Rai, Spectrochim. Acta. A91 (2012)
217
251. S.K. Singh, A.
Chaudhary, D.K. Rai and S.B. Rai, Ind. J. Trad. Knowl, 8 (2009) 346
252. S.K. Singh, S.K. Jha,
A.K. Chaudhary, R.D.S. Yadav and S.B. Rai, Pharma. Bio. 48 (2010) 134
253. S.K. Singh, D.N.S.
Gautam, M.Kumar and S.B. Rai, Ind. J. Pharma. Sci 72 (2010) 24
254. S.K. Singh and S.B.
Rai, Ind. J. Pharma. Sci. 74 (2012)
178
255. S.K. Goel, M.J. Singh and R. Shanker, PRAMANA 45 (1995) 291
256. R. Shanker and S.K. Goel, Ind. J. Phys. 71B (1997) 363
257. S.K. Goel, M.J. Singh and R. Shanker, Phys, Rev. A52 (1995) 2453
258. S.K. Goel, M.J. Singh and R. Shanker, Phys, Rev. A54 (1996) 2056
259. B. Bapat, E. Krishnakumar, C.P. Safvan, M.J. Singh, S.K. Goel and
R.Shanker Phys. Rev. A54 (1996) 2959
260.M.J. Singh, S.K. Goel, R. Shanker, D.O. Kataria, N. Madhavan,
J.J. Das, D.K. Awasthi, P. Sugathan and A.K. Sinha, PRAMANA 49 (1997) 521
261. R. Shanker and R. Hipler, Z. Phys. D42 (1997) 161
262. S.K. Goel and R. Shanker, J. X-ray Sci. and Tech. 7 (1997) 331
263. S.K. Goel, M.J. Singh and R. Shanker, Ind. J. Phys. 72A (1998) 65
264. S.K. Goel, R. Hippler, R.K. Singh and R. Shanker, PRAMANA 52 (1999) 493
265. M.J. Singh, R. Shanker, D.O. Kataria, N. Madhvan, P.Sugathan,
J.J, Das, D.K. Awasthi and A.K. Sinha, PRAMANA 53 (1999) 743
266. R.K. Singh, R.K. Mohanta. M.J. Singh, R. Hippler and R. Shanker,
PRAMANA 58 (2002) 631
267. R.K. Singh, R.K. Mohanta, R. Hippler and R. Shanker, PRAMANA 58
(2002) 499
268. R.K. Singh, R. Hippler
and R. Shanker, J. Phys. B35 (2002)
3243
269. R.K. Singh, R. Hippler
and R. Shanker, Phys. Rev. A67 (2003)
022704
270. S. Mondal, R.K. Singh
and R. Shanker, PRAMANA 60 (2003)
1203
271. R.K. Singh, S. Mondal
and R. Shanker, J. Phys. B36 (2003)
489
272. R.K. Singh and R.
Shanker, J. Phys. B36 (2003) 1545
273. R.K.Yadav, Argala
Srivastava, S.Mondal and R. Shanker, J. Phys. D36 (2003) 2538
274. R.K. Yadav and R.
Shanker, Phys. Rev. A70 (2004)
052901
275. R.K. Yadav and R.
Shanker, Ind. J. Phys. 80 (2006) 43
276. R.K. Yadav and R.
Shanker, PRAMANA 68 (2007) 507
277. A.N. Agnihotri, V.S.
Subrahmanyam, R.K. Yadav, X. Lovet and R. Shanker, J. Phys. D41 (2008) 065205
278. Namita Yadav, Pragya
Bhatt, R. Singh, V.S. Subrahmanyam and R. Shanker, PRAMANA 74 (2010) 563
279. Pragya Bhatt, R.
Singh, Namita Yadav and R. Shanker, Phys. Rev. A82 (2010) 044702
280. Pragya Bhatt, R.
Singh, Namita Yadav and R. Shanker, Phys. Rev. A84 (2011) 012701
281. Pragya Bhatt, R.
Singh, Namita Yadav and R. Shanker, Phys. Rev. A85 (2012) 034702
282. Pragya Bhatt, R.
Singh, Namita Yadav and R. Shanker, Phys. Rev. A86 (2012) 052708
283. Pragya Bhatt, R.
Singh, Namita Yadav and R. Shanker, Phys. Rev. A85 (2012) 042707
284. V.K.
Singh, V. Singh, A.K. Rai, S.N. Thakur, P.K. Rai and J.P. Singh, Appl. Opt. 47
(2008) G-38-47
285. Shikha
Rai, A.K. Rai and S.N. Thakur, Appl. Phys. B91 (2008) 645
286. N.K.
Rai, A.K. Rai, A. Kumar and S.N. Thakur, Appl. Opt. 47 (2009) G-105-111
287. Y.
Dwivedi, S.N. Thakur and S.B. Rai, Appl. Opt. 49 (2010) C-42
288. Shiwani
Pandhija, N.K. Rai, A.K. Rai and S.N. Thakur, Appl. Phys. B98 (2010) 231
oooooOOOOOooooo
|